Triptorelin pamoate: Difference between revisions
Gloria Picoy (talk | contribs) No edit summary |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 62: | Line 62: | ||
Human pharmacokinetic data with triptorelin suggest that C-terminal fragments produced by tissue degradation are either degraded completely within tissues or are rapidly degraded further in plasma, or cleared by the kidneys. Therefore, hepatic microsomal enzymes are unlikely to be involved in triptorelin metabolism. However, in the absence of relevant data and as a precaution, hyperprolactinemic drugs should not be used concomitantly with triptorelin since hyperprolactinemia reduces the number of pituitary GnRH receptors. | Human pharmacokinetic data with triptorelin suggest that C-terminal fragments produced by tissue degradation are either degraded completely within tissues or are rapidly degraded further in plasma, or cleared by the kidneys. Therefore, hepatic microsomal enzymes are unlikely to be involved in triptorelin metabolism. However, in the absence of relevant data and as a precaution, hyperprolactinemic drugs should not be used concomitantly with triptorelin since hyperprolactinemia reduces the number of pituitary GnRH receptors. | ||
|FDAPregCat=X | |FDAPregCat=X | ||
|useInPregnancyFDA=Triptorelin pamoate is contraindicated in women who are or may become pregnant while receiving the drug. Expected hormonal changes that occur with TRELSTAR treatment increase the risk for pregnancy loss. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. | |||
Studies in pregnant rats administered triptorelin at doses of 2, 10, and 100 mcg/kg/day (approximately equivalent to 0.2, 0.8, and 8 times the estimated human daily dose based on body surface area) during the period of organogenesis demonstrated maternal toxicity and embryo-fetal toxicities. Embryo-fetal toxicities consisted of pre-implantation loss, increased resorption, and reduced mean number of viable fetuses at the high dose. Teratogenic effects were not observed in viable fetuses in rats or mice. Doses administered to mice were 2, 20, and 200 mcg/kg/day (approximately equivalent to 0.1, 0.7, and 7 times the estimated human daily dose based on body surface area). | |||
|useInNursing=Triptorelin pamoate is not indicated for use in women. It is not known if triptorelin is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from TRELSTAR, a decision should be made to either discontinue nursing, or discontinue the drug taking into account the importance of the drug to the mother. | |||
|useInPed=Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri=Prostate cancer occurs primarily in an older population. Clinical studies with TRELSTAR have been conducted primarily in patients ≥ 65 years. | |||
|useInRace=The effects of race on triptorelin pharmacokinetics have not been systematically studied. | |||
|useInRenalImpair=Subjects with renal impairment had higher exposure than young healthy males. | |||
|useInHepaticImpair=Subjects with hepatic impairment had higher exposure than young healthy males. | |||
|useInReproPotential=After 60 days of subcutaneous treatment followed by a minimum of four estrus cycles prior to mating, triptorelin, at doses of 2, 20, and 200 mcg/kg/day in saline (approximately 0.2, 2, and 16 times the estimated human daily dose based on body surface area) or 2 monthly injections as slow release microspheres (~20 mcg/kg/day), had no effect on the fertility or general reproductive function of female rats. | |||
No studies were conducted to assess the effect of triptorelin on male fertility. | |||
|administration=Intramuscular | |administration=Intramuscular | ||
|overdose=There is no experience of overdosage in clinical trials. In single dose toxicity studies in mice and rats, the subcutaneous LD50 of triptorelin was 400 mg/kg in mice and 250 mg/kg in rats, approximately 500 and 600 times, respectively, the estimated monthly human dose based on body surface area. If overdosage occurs, therapy should be discontinued immediately and the appropriate supportive and symptomatic treatment administered. | |||
|mechAction=Triptorelin is a synthetic decapeptide agonist analog of gonadotropin releasing hormone (GnRH). Comparative in vitro studies showed that triptorelin was 100-fold more active than native GnRH in stimulating luteinizing hormone release from monolayers of dispersed rat pituitary cells in culture and 20-fold more active than native GnRH in displacing 125I-GnRH from pituitary receptor sites. In animal studies, triptorelin pamoate was found to have 13‑fold higher luteinizing hormone-releasing activity and 21-fold higher follicle-stimulating hormone-releasing activity compared to the native GnRH. | |||
|structure=The structural formula is: | |||
[[File:Triptorelin pamoate structural formula.png|thumb|none|500px]] | |||
|PD=Following the first administration, there is a transient surge in circulating levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, and estradiol [see ADVERSE REACTIONS (6)]. After chronic and continuous administration, usually 2 to 4 weeks after initiation of therapy, a sustained decrease in LH and FSH secretion and marked reduction of testicular steroidogenesis are observed. A reduction of serum testosterone concentration to a level typically seen in surgically castrated men is obtained. Consequently, the result is that tissues and functions that depend on these hormones for maintenance become quiescent. These effects are usually reversible after cessation of therapy. | |||
|PK=Results of pharmacokinetic investigations conducted in healthy men indicate that after intravenous bolus administration, triptorelin is distributed and eliminated according to a 3-compartment model and corresponding half-lives are approximately 6 minutes, 45 minutes, and 3 hours. | |||
======Absorption====== | |||
Following a single intramuscular injection of TRELSTAR to patients with prostate cancer, mean peak serum concentrations of 28.4 ng/mL, 38.5 ng/mL, and 44.1 ng/mL occurred in 1 to 3 hours after the 3.75 mg, 11.25 mg, and 22.5 mg formulations, respectively. | |||
Triptorelin did not accumulate over 9 months (3.75 mg and 11.25 mg) or 12 months (22.5 mg) of treatment. | |||
======Distribution====== | |||
The volume of distribution following a single intravenous bolus dose of 0.5 mg of triptorelin peptide was 30 – 33 L in healthy male volunteers. There is no evidence that triptorelin, at clinically relevant concentrations, binds to plasma proteins. | |||
======Metabolism====== | |||
The metabolism of triptorelin in humans is unknown, but is unlikely to involve hepatic microsomal enzymes (cytochrome P-450). The effect of triptorelin on the activity of other drug metabolizing enzymes is also unknown. Thus far, no metabolites of triptorelin have been identified. Pharmacokinetic data suggest that C-terminal fragments produced by tissue degradation are either completely degraded in the tissues, or rapidly degraded in plasma, or cleared by the kidneys. | |||
======Excretion====== | |||
Triptorelin is eliminated by both the liver and the kidneys. Following intravenous administration of 0.5 mg triptorelin peptide to six healthy male volunteers with a creatinine clearance of 149.9 mL/min, 41.7% of the dose was excreted in urine as intact peptide with a total triptorelin clearance of 211.9 mL/min. This percentage increased to 62.3% in patients with liver disease who have a lower creatinine clearance (89.9 mL/min). It has also been observed that the nonrenal clearance of triptorelin (patient anuric, CIcreat = 0) was 76.2 mL/min, thus indicating that the nonrenal elimination of triptorelin is mainly dependent on the liver. | |||
|nonClinToxic=======Carcinogenesis and Mutagenesis====== | |||
In rats, doses of 120, 600, and 3000 mcg/kg given every 28 days (approximately 0.3, 2, and 8 times the human monthly dose based on body surface area) resulted in increased mortality with a drug treatment period of 13 – 19 months. The incidences of benign and malignant pituitary tumors and histiosarcomas were increased in a dose-related manner. No oncogenic effect was observed in mice administered triptorelin for 18 months at doses up to 6000 mcg/kg every 28 days (approximately 8 times the human monthly dose based on body surface area). | |||
Mutagenicity studies performed with triptorelin using bacterial and mammalian systems (in vitro Ames test and chromosomal aberration test in CHO cells and an in vivo mouse micronucleus test) provided no evidence of mutagenic potential. | |||
|alcohol=Alcohol-Triptorelin pamoate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Triptorelin pamoate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* Trelstar Depot | |brandNames=* Trelstar Depot | ||
Revision as of 06:12, 28 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Triptorelin pamoate is a gonadotropin releasing hormone (GnRH) agonist that is FDA approved for the {{{indicationType}}} of advanced prostate cancer. Common adverse reactions include hot flushes, skeletal pain, impotence and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Triptorelin pamoate is indicated for the palliative treatment of advanced prostate cancer.
- Dosage:
- Triptorelin pamoate 3.75 mg, 1 injection every 4 weeks
- Triptorelin pamoate 11.25 mg, 1 injection every 12 weeks
- Triptorelin pamoate 22.5 mg, 1 injection every 24 weeks
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Triptorelin pamoate in adult patients.
Non–Guideline-Supported Use
- Central precocious puberty
- Endometrial hyperplasia
- Endometriosis
- Fibrocystic breast changes
- In vitro fertilization
- Uterine leiomyoma
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness not established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Triptorelin pamoate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Triptorelin pamoate in pediatric patients.
Contraindications
Hypersensitivity
TRELSTAR is contraindicated in individuals with a known hypersensitivity to triptorelin or any other component of the product, or other GnRH agonists or GnRH.
Pregnancy
Triptorelin pamoate may cause fetal harm when administered to a pregnant woman. Expected hormonal changes that occur with TRELSTAR treatment increase the risk for pregnancy loss and fetal harm when administered to a pregnant woman. Triptorelin pamoate is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Warnings
Hypersensitivity Reactions
Anaphylactic shock, hypersensitivity, and angioedema related to triptorelin administration have been reported. In the event of a hypersensitivity reaction, therapy with TRELSTAR should be discontinued immediately and the appropriate supportive and symptomatic care should be administered.
Transient Increase in Serum Testosterone
Initially, triptorelin, like other GnRH agonists, causes a transient increase in serum testosterone levels. As a result, isolated cases of worsening of signs and symptoms of prostate cancer during the first weeks of treatment have been reported with GnRH agonists. Patients may experience worsening of symptoms or onset of new symptoms, including bone pain, neuropathy, hematuria, or urethral or bladder outlet obstruction.
Metastatic Vertebral Lesions and Urinary Tract Obstruction
Cases of spinal cord compression, which may contribute to weakness or paralysis with or without fatal complications, have been reported with GnRH agonists. If spinal cord compression or renal impairment develops, standard treatment of these complications should be instituted, and in extreme cases an immediate orchiectomy considered.
Patients with metastatic vertebral lesions and/or with upper or lower urinary tract obstruction should be closely observed during the first few weeks of therapy.
Effect on QT/QTc Interval
Androgen deprivation therapy may prolong the QT/QTc interval. Providers should consider whether the benefits of androgen deprivation therapy outweigh the potential risks in patients with congenital long QT syndrome, congestive heart failure, frequent electrolyte abnormalities, and in patients taking drugs known to prolong the QT interval. Electrolyte abnormalities should be corrected. Consider periodic monitoring of electrocardiograms and electrolytes.
Hyperglycemia and Diabetes
Hyperglycemia and an increased risk of developing diabetes have been reported in men receiving GnRH agonists. Hyperglycemia may represent development of diabetes mellitus or worsening of glycemic control in patients with diabetes. Monitor blood glucose and/or glycosylated hemoglobin (HbA1c) periodically in patients receiving a GnRH agonist and manage with current practice for treatment of hyperglycemia or diabetes.
Cardiovascular Diseases
Increased risk of developing myocardial infarction, sudden cardiac death and stroke has been reported in association with use of GnRH agonists in men. The risk appears low based on the reported odds ratios, and should be evaluated carefully along with cardiovascular risk factors when determining a treatment for patients with prostate cancer. Patients receiving a GnRH agonist should be monitored for symptoms and signs suggestive of development of cardiovascular disease and be managed according to current clinical practice.
Laboratory Tests
Response to TRELSTAR should be monitored by measuring serum levels of testosterone periodically or as indicated.
Laboratory Test Interactions
Chronic or continuous administration of triptorelin in therapeutic doses results in suppression of pituitary-gonadal axis. Diagnostic tests of the pituitary-gonadal function conducted during treatment and after cessation of therapy may therefore be misleading.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Triptorelin pamoate Clinical Trials Experience in the drug label.
Postmarketing Experience
The following adverse reactions have been identified during post approval use of gonadotropin releasing hormone agonists. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
During postmarketing surveillance, rare cases of pituitary apoplexy (a clinical syndrome secondary to infarction of the pituitary gland) have been reported after the administration of gonadotropin-releasing hormone agonists. In a majority of these cases, a pituitary adenoma was diagnosed with a majority of pituitary apoplexy cases occurring within 2 weeks of the first dose, and some within the first hour. In these cases, pituitary apoplexy has presented as sudden headache, vomiting, visual changes, ophthalmoplegia, altered mental status, and sometimes cardiovascular collapse. Immediate medical attention has been required.
During postmarketing experience, convulsions, and thromboembolic events including, but not limited to, pulmonary emboli, cerebrovascular accident, myocardial infarction, deep venous thrombosis, transient ischemic attack, and thrombophlebitis have been reported.
Drug Interactions
No drug-drug interaction studies involving triptorelin have been conducted.
Human pharmacokinetic data with triptorelin suggest that C-terminal fragments produced by tissue degradation are either degraded completely within tissues or are rapidly degraded further in plasma, or cleared by the kidneys. Therefore, hepatic microsomal enzymes are unlikely to be involved in triptorelin metabolism. However, in the absence of relevant data and as a precaution, hyperprolactinemic drugs should not be used concomitantly with triptorelin since hyperprolactinemia reduces the number of pituitary GnRH receptors.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): X Triptorelin pamoate is contraindicated in women who are or may become pregnant while receiving the drug. Expected hormonal changes that occur with TRELSTAR treatment increase the risk for pregnancy loss. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
Studies in pregnant rats administered triptorelin at doses of 2, 10, and 100 mcg/kg/day (approximately equivalent to 0.2, 0.8, and 8 times the estimated human daily dose based on body surface area) during the period of organogenesis demonstrated maternal toxicity and embryo-fetal toxicities. Embryo-fetal toxicities consisted of pre-implantation loss, increased resorption, and reduced mean number of viable fetuses at the high dose. Teratogenic effects were not observed in viable fetuses in rats or mice. Doses administered to mice were 2, 20, and 200 mcg/kg/day (approximately equivalent to 0.1, 0.7, and 7 times the estimated human daily dose based on body surface area).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Triptorelin pamoate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Triptorelin pamoate during labor and delivery.
Nursing Mothers
Triptorelin pamoate is not indicated for use in women. It is not known if triptorelin is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from TRELSTAR, a decision should be made to either discontinue nursing, or discontinue the drug taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Prostate cancer occurs primarily in an older population. Clinical studies with TRELSTAR have been conducted primarily in patients ≥ 65 years.
Gender
There is no FDA guidance on the use of Triptorelin pamoate with respect to specific gender populations.
Race
The effects of race on triptorelin pharmacokinetics have not been systematically studied.
Renal Impairment
Subjects with renal impairment had higher exposure than young healthy males.
Hepatic Impairment
Subjects with hepatic impairment had higher exposure than young healthy males.
Females of Reproductive Potential and Males
After 60 days of subcutaneous treatment followed by a minimum of four estrus cycles prior to mating, triptorelin, at doses of 2, 20, and 200 mcg/kg/day in saline (approximately 0.2, 2, and 16 times the estimated human daily dose based on body surface area) or 2 monthly injections as slow release microspheres (~20 mcg/kg/day), had no effect on the fertility or general reproductive function of female rats.
No studies were conducted to assess the effect of triptorelin on male fertility.
Immunocompromised Patients
There is no FDA guidance one the use of Triptorelin pamoate in patients who are immunocompromised.
Administration and Monitoring
Administration
Intramuscular
Monitoring
There is limited information regarding Triptorelin pamoate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Triptorelin pamoate and IV administrations.
Overdosage
There is no experience of overdosage in clinical trials. In single dose toxicity studies in mice and rats, the subcutaneous LD50 of triptorelin was 400 mg/kg in mice and 250 mg/kg in rats, approximately 500 and 600 times, respectively, the estimated monthly human dose based on body surface area. If overdosage occurs, therapy should be discontinued immediately and the appropriate supportive and symptomatic treatment administered.
Pharmacology
There is limited information regarding Triptorelin pamoate Pharmacology in the drug label.
Mechanism of Action
Triptorelin is a synthetic decapeptide agonist analog of gonadotropin releasing hormone (GnRH). Comparative in vitro studies showed that triptorelin was 100-fold more active than native GnRH in stimulating luteinizing hormone release from monolayers of dispersed rat pituitary cells in culture and 20-fold more active than native GnRH in displacing 125I-GnRH from pituitary receptor sites. In animal studies, triptorelin pamoate was found to have 13‑fold higher luteinizing hormone-releasing activity and 21-fold higher follicle-stimulating hormone-releasing activity compared to the native GnRH.
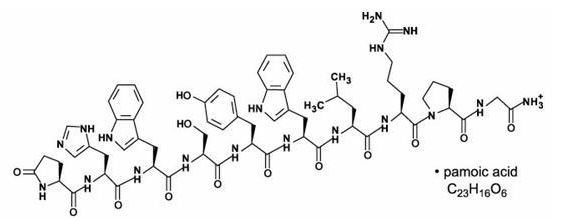
Structure
The structural formula is:
Pharmacodynamics
Following the first administration, there is a transient surge in circulating levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone, and estradiol [see ADVERSE REACTIONS (6)]. After chronic and continuous administration, usually 2 to 4 weeks after initiation of therapy, a sustained decrease in LH and FSH secretion and marked reduction of testicular steroidogenesis are observed. A reduction of serum testosterone concentration to a level typically seen in surgically castrated men is obtained. Consequently, the result is that tissues and functions that depend on these hormones for maintenance become quiescent. These effects are usually reversible after cessation of therapy.
Pharmacokinetics
Results of pharmacokinetic investigations conducted in healthy men indicate that after intravenous bolus administration, triptorelin is distributed and eliminated according to a 3-compartment model and corresponding half-lives are approximately 6 minutes, 45 minutes, and 3 hours.
Absorption
Following a single intramuscular injection of TRELSTAR to patients with prostate cancer, mean peak serum concentrations of 28.4 ng/mL, 38.5 ng/mL, and 44.1 ng/mL occurred in 1 to 3 hours after the 3.75 mg, 11.25 mg, and 22.5 mg formulations, respectively.
Triptorelin did not accumulate over 9 months (3.75 mg and 11.25 mg) or 12 months (22.5 mg) of treatment.
Distribution
The volume of distribution following a single intravenous bolus dose of 0.5 mg of triptorelin peptide was 30 – 33 L in healthy male volunteers. There is no evidence that triptorelin, at clinically relevant concentrations, binds to plasma proteins.
Metabolism
The metabolism of triptorelin in humans is unknown, but is unlikely to involve hepatic microsomal enzymes (cytochrome P-450). The effect of triptorelin on the activity of other drug metabolizing enzymes is also unknown. Thus far, no metabolites of triptorelin have been identified. Pharmacokinetic data suggest that C-terminal fragments produced by tissue degradation are either completely degraded in the tissues, or rapidly degraded in plasma, or cleared by the kidneys.
Excretion
Triptorelin is eliminated by both the liver and the kidneys. Following intravenous administration of 0.5 mg triptorelin peptide to six healthy male volunteers with a creatinine clearance of 149.9 mL/min, 41.7% of the dose was excreted in urine as intact peptide with a total triptorelin clearance of 211.9 mL/min. This percentage increased to 62.3% in patients with liver disease who have a lower creatinine clearance (89.9 mL/min). It has also been observed that the nonrenal clearance of triptorelin (patient anuric, CIcreat = 0) was 76.2 mL/min, thus indicating that the nonrenal elimination of triptorelin is mainly dependent on the liver.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
In rats, doses of 120, 600, and 3000 mcg/kg given every 28 days (approximately 0.3, 2, and 8 times the human monthly dose based on body surface area) resulted in increased mortality with a drug treatment period of 13 – 19 months. The incidences of benign and malignant pituitary tumors and histiosarcomas were increased in a dose-related manner. No oncogenic effect was observed in mice administered triptorelin for 18 months at doses up to 6000 mcg/kg every 28 days (approximately 8 times the human monthly dose based on body surface area).
Mutagenicity studies performed with triptorelin using bacterial and mammalian systems (in vitro Ames test and chromosomal aberration test in CHO cells and an in vivo mouse micronucleus test) provided no evidence of mutagenic potential.
Clinical Studies
There is limited information regarding Triptorelin pamoate Clinical Studies in the drug label.
How Supplied
There is limited information regarding Triptorelin pamoate How Supplied in the drug label.
Storage
There is limited information regarding Triptorelin pamoate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Triptorelin pamoate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Triptorelin pamoate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Triptorelin pamoate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Triptorelin pamoate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Trelstar Depot
- Trelstar LA
- Trelstar [1]
Look-Alike Drug Names
There is limited information regarding Triptorelin pamoate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.