Tranexamic acid (oral): Difference between revisions
No edit summary |
No edit summary |
||
| Line 79: | Line 79: | ||
A case of severe allergic reaction to LYSTEDA was reported in the extension trial, involving a subject on her fourth cycle of treatment, who experienced dyspnea, tightening of her throat, and facial flushing that required emergency medical treatment. | A case of severe allergic reaction to LYSTEDA was reported in the extension trial, involving a subject on her fourth cycle of treatment, who experienced dyspnea, tightening of her throat, and facial flushing that required emergency medical treatment. | ||
|drugInteractions=7.1 Hormonal Contraceptives | |||
Because LYSTEDA is antifibrinolytic, concomitant use of hormonal contraception and LYSTEDA may further exacerbate the increased thrombotic risk associated with combination hormonal contraceptives. For this reason, concomitant use of LYSTEDA with combination hormonal contraceptives is contraindicated [see CONTRAINDICATIONS (4) and WARNINGS AND PRECAUTIONS (5.1)]. | |||
7.2 Tissue Plasminogen Activators | |||
Concomitant therapy with tissue plasminogen activators may decrease the efficacy of both LYSTEDA and tissue plasminogen activators. Therefore, exercise caution if a woman taking LYSTEDA therapy requires tissue plasminogen activators. | |||
7.3 Factor IX Complex Concentrates or Anti-Inhibitor Coagulant Concentrates | |||
LYSTEDA is not recommended for women taking either Factor IX complex concentrates or anti-inhibitor coagulant concentrates because the risk of thrombosis may be increased [see WARNINGS AND PRECAUTIONS (5.1) and CLINICAL PHARMACOLOGY (12.3)]. | |||
7.4 ALL-TRANS RETINOIC ACID (ORAL TRETINOIN) | |||
Exercise caution when prescribing LYSTEDA to women with acute promyelocytic leukemia taking all-trans retinoic acid for remission induction because of possible exacerbation of the procoagulant effect of all-trans retinoic acid [see WARNINGS AND PRECAUTIONS (5.1) and CLINICAL PHARMACOLOGY (12.3)]. | |||
|FDAPregCat=B | |||
|useInPregnancyFDA=LYSTEDA is not indicated for use in pregnant women. Reproduction studies have been performed in mice, rats and rabbits and have revealed no evidence of impaired fertility or harm to the fetus due to tranexamic acid. However, tranexamic acid is known to cross the placenta and appears in cord blood at concentrations approximately equal to the maternal concentration. There are no adequate and well-controlled studies in pregnant women [see NONCLINICAL TOXICOLOGY (13.1)]. | |||
An embryo-fetal developmental toxicity study in rats and a perinatal developmental toxicity study in rats were conducted using tranexamic acid. No adverse effects were observed in either study at doses up to 4 times the recommended human oral dose of 3900 mg/day based on mg/m2 (actual animal dose 1500 mg/kg/day). | |||
|useInNursing=Tranexamic acid is present in the mother's milk at a concentration of about one hundredth of the corresponding serum concentration. LYSTEDA should be used during lactation only if clearly needed. | |||
|useInPed=LYSTEDA is indicated for women of reproductive age and is not intended for use in premenarcheal girls. | |||
Based on a pharmacokinetic study in 20 adolescent females, 12 to 16 years of age, no dose adjustment is needed in the adolescent population. | |||
|useInGeri=LYSTEDA is indicated for women of reproductive age and is not intended for use by postmenopausal women. | |||
|useInRenalImpair=The effect of renal impairment on the pharmacokinetics of LYSTEDA has not been studied. Because tranexamic acid is primarily eliminated via the kidneys by glomerular filtration with more than 95% excreted as unchanged in urine, dosage adjustment in patient with renal impairment is needed [see DOSAGE AND ADMINISTRATION (2.2) and CLINICAL PHARMACOLOGY (12.3)]. | |||
|useInHepaticImpair=The effect of hepatic impairment on the pharmacokinetics of LYSTEDA has not been studied. Because only a small fraction of the drug is metabolized, dosage adjustment in patients with hepatic impairment is not needed [see CLINICAL PHARMACOLOGY (12.3)]. | |||
|overdose=There are no known cases of intentional overdose with LYSTEDA and no subjects in the clinical program took more than 2 times the prescribed amount of LYSTEDA in a 24-hour period (>7800 mg/day). However, cases of overdose of tranexamic acid have been reported. Based on these reports, symptoms of overdose may include gastrointestinal (nausea, vomiting, diarrhea); hypotensive (e.g., orthostatic symptoms); thromboembolic (arterial, venous, embolic); visual impairment; mental status changes; myoclonus; or rash. No specific information is available on the treatment of overdose with LYSTEDA. In the event of overdose, employ the usual supportive measures (e.g., clinical monitoring and supportive therapy) as dictated by the patient's clinical status. | |||
|drugBox={{Drugbox2 | |||
| verifiedrevid = 477001216 | |||
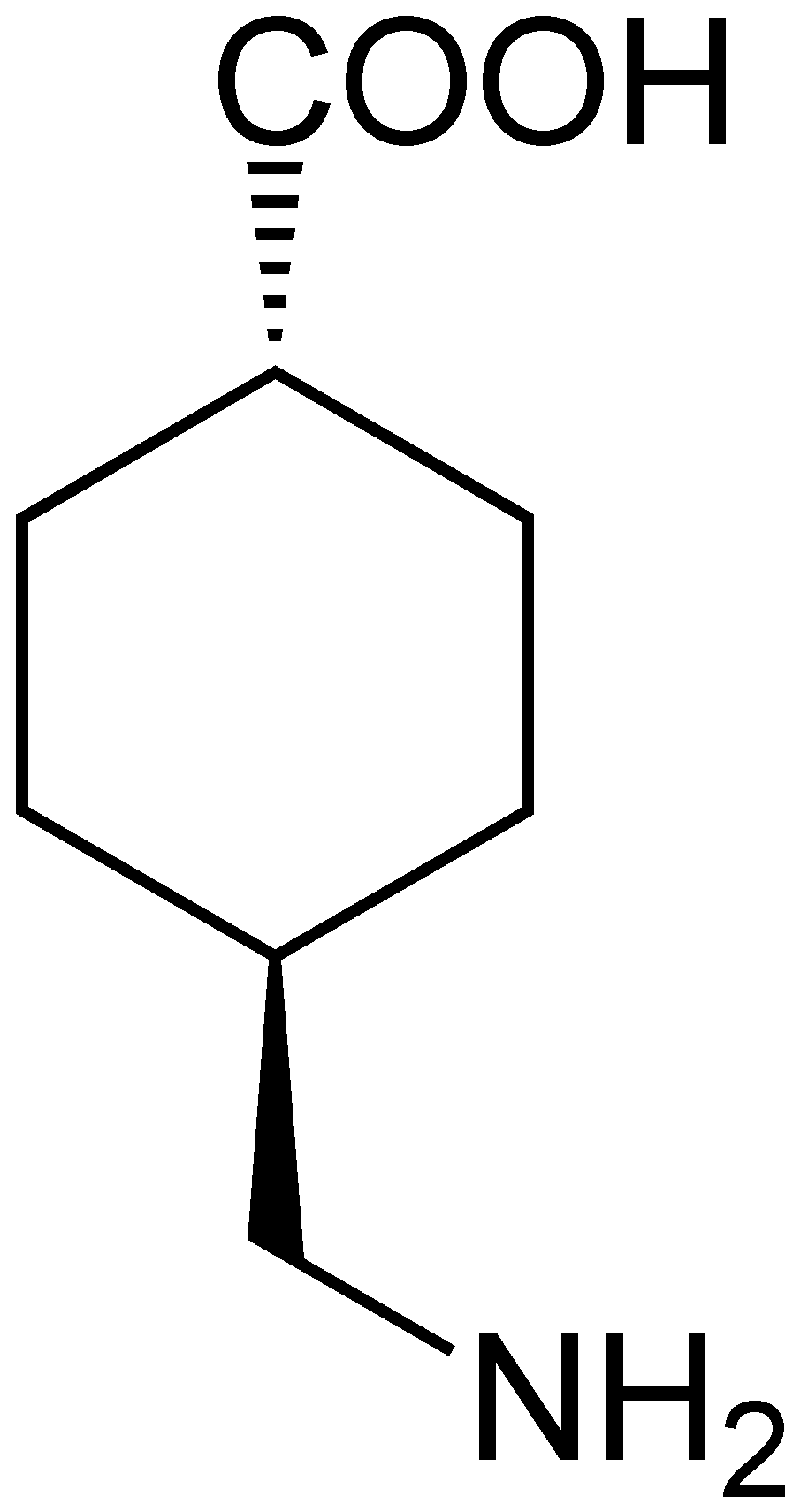
| IUPAC_name = ''trans''-4-(aminomethyl)[[cyclohexanecarboxylic acid]] | |||
| image = Tranexamic acid Structural Formulae.png | |||
| width = 110 | |||
| image2 = Tranexamic acid ball-and-stick.png | |||
| width2 = 200 | |||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|CDI|tranexamic_acid}} | |||
| pregnancy_category = B | |||
| legal_status = P (UK) | |||
| routes_of_administration = Injection and oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 34% | |||
| elimination_half-life = 3.1 h | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 1197-18-8 | |||
| ATC_prefix = B02 | |||
| ATC_suffix = AA02 | |||
| PubChem = 5526 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00302 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 10482000 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 6T84R30KC1 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D01136 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 48669 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 877 | |||
<!--Chemical data--> | |||
| C=8 | H=15 | N=1 | O=2 | |||
| molecular_weight = 157.21 g/mol | |||
| smiles = NC[C@@H]1CC[C@H](CC1)C(O)=O | |||
| InChI = 1/C8H15NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h6-7H,1-5,9H2,(H,10,11)/t6-,7- | |||
| InChIKey = GYDJEQRTZSCIOI-LJGSYFOKBE | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C8H15NO2/c9-5-6-1-3-7(4-2-6)8(10)11/h6-7H,1-5,9H2,(H,10,11)/t6-,7- | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = GYDJEQRTZSCIOI-LJGSYFOKSA-N | |||
}} | |||
|structure=LYSTEDA is an antifibrinolytic drug. The chemical name is trans-4-aminomethyl-cyclohexanecarboxylic acid. The structural formula is: | |||
[[File:Tranexamic acid2.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Tranexamic acid is a white crystalline powder. It is freely soluble in water and in glacial acetic acid and is very slightly soluble in ethanol and practically insoluble in ether. The molecular formula is C8H15N02 and the molecular weight is 157.2. | |||
Tranexamic acid tablets are provided as white oval-shaped tablets and are not scored. Each tablet is debossed with the marking "FP650." The active ingredient in each tablet is 650 mg tranexamic acid. The inactive ingredients contained in each tablet are: microcrystalline cellulose, colloidal silicon dioxide, pregelatinized corn starch, povidone, hypromellose, stearic acid, and magnesium stearate. | |||
|alcohol=Alcohol-Tranexamic acid (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Tranexamic acid (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 14:00, 11 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tranexamic acid (oral) is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the treatment of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indicationsd
LYSTEDA™ (tranexamic acid) Tablets is indicated for the treatment of cyclic heavy menstrual bleeding [see CLINICAL STUDIES (14)].
Prior to prescribing LYSTEDA, exclude endometrial pathology that can be associated with heavy menstrual bleeding.
Dosage
2.1 Recommended Dosage The recommended dose of LYSTEDA for women with normal renal function is two 650 mg tablets taken three times daily (3900 mg/day) for a maximum of 5 days during monthly menstruation. LYSTEDA may be administered without regard to meals. Tablets should be swallowed whole and not chewed or broken apart.
2.2 Renal Impairment In patients with renal impairment, the plasma concentration of tranexamic acid increased as serum creatinine concentration increased [see CLINICAL PHARMACOLOGY (12.3)]. Dosage adjustment is needed in patients with serum creatinine concentration higher than 1.4 mg/dL (Table 1).

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tranexamic acid (oral) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tranexamic acid (oral) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Tranexamic acid (oral) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tranexamic acid (oral) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tranexamic acid (oral) in pediatric patients.
Contraindications
4.1 Thromboembolic Risk Do not prescribe LYSTEDA to women who are
using combination hormonal contraception known to have any of the following conditions: Active thromboembolic disease (e.g., deep vein thrombosis, pulmonary embolism, or cerebral thrombosis) A history of thrombosis or thromboembolism, including retinal vein or artery occlusion An intrinsic risk of thrombosis or thromboembolism (e.g., thrombogenic valvular disease, thrombogenic cardiac rhythm disease, or hypercoagulopathy) Venous and arterial thrombosis or thromboembolism, as well as cases of retinal artery and retinal vein occlusions, have been reported with tranexamic acid.
4.2 Hypersensitivity to Tranexamic Acid Do not prescribe LYSTEDA to women with known hypersensitivity to tranexamic acid
Warnings
5.1 Thromboembolic Risk Concomitant Use of Hormonal Contraceptives
Combination hormonal contraceptives are known to increase the risk of venous thromboembolism, as well as arterial thromboses such as stroke and myocardial infarction. Because LYSTEDA is antifibrinolytic, the risk of venous thromboembolism, as well as arterial thromboses such as stroke, may increase further when hormonal contraceptives are administered with LYSTEDA. This is of particular concern in women who are obese or smoke cigarettes, especially smokers over 35 years of age.
Women using hormonal contraception were excluded from the clinical trials supporting the safety and efficacy of LYSTEDA, and there are no clinical trial data on the risk of thrombotic events with the concomitant use of LYSTEDA with hormonal contraceptives. However, there have been US postmarketing reports of venous and arterial thrombotic events in women who have used LYSTEDA concomitantly with combination hormonal contraceptives. For this reason, concomitant use of LYSTEDA with combination hormonal contraceptives is contraindicated. [see CONTRAINDICATIONS (4.1) and DRUG INTERACTIONS (7.1)].
Factor IX Complex Concentrates or Anti-Inhibitor Coagulant Concentrates
LYSTEDA is not recommended for women taking either Factor IX complex concentrates or anti-inhibitor coagulant concentrates because the risk of thrombosis may be increased [see DRUG INTERACTIONS (7.3) and CLINICAL PHARMACOLOGY (12.3)].
All-Trans Retinoic Acid (Oral Tretinoin)
Exercise caution when prescribing LYSTEDA to women with acute promyelocytic leukemia taking all-trans retinoic acid for remission induction because of possible exacerbation of the procoagulant effect of all-trans retinoic acid [see DRUG INTERACTIONS (7.4) and CLINICAL PHARMACOLOGY (12.3)].
Ocular Effects
Retinal venous and arterial occlusion has been reported in patients using tranexamic acid. Patients should be instructed to report visual and ocular symptoms promptly. In the event of such symptoms, patients should be instructed to discontinue LYSTEDA immediately and should be referred to an ophthalmologist for a complete ophthalmic evaluation, including dilated retinal examination, to exclude the possibility of retinal venous or arterial occlusion.
5.2 Severe Allergic Reaction A case of severe allergic reaction to LYSTEDA was reported in the clinical trials, involving a subject who experienced dyspnea, tightening of her throat, and facial flushing that required emergency medical treatment. A case of anaphylactic shock has also been reported in the literature, involving a patient who received an intravenous bolus of tranexamic acid.
5.3 Subarachnoid Hemorrhage Cerebral edema and cerebral infarction may be caused by use of LYSTEDA in women with subarachnoid hemorrhage.
5.4 Ligneous Conjunctivitis Ligneous conjunctivitis has been reported in patients taking tranexamic acid. The conjunctivitis resolved following cessation of the drug.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Short-term Studies
The safety of LYSTEDA in the treatment of heavy menstrual bleeding (HMB) was studied in two randomized, double-blind, placebo-controlled studies [see CLINICAL STUDIES (14)]. One study compared the effects of two doses of LYSTEDA (1950 mg and 3900 mg given daily for up to 5 days during each menstrual period) versus placebo over a 3-cycle treatment duration. A total of 304 women were randomized to this study, with 115 receiving at least one dose of 3900 mg/day of LYSTEDA. A second study compared the effects of LYSTEDA (3900 mg/day) versus placebo over a 6-cycle treatment duration. A total of 196 women were randomized to this study, with 117 receiving at least one dose of LYSTEDA. In both studies, subjects were generally healthy women who had menstrual blood loss of ≥ 80 mL.
In these studies, subjects were 18 to 49 years of age with a mean age of approximately 40 years, had cyclic menses every 21-35 days, and a BMI of approximately 32 kg/m2. On average, subjects had a history of HMB for approximately 10 years and 40% had fibroids as determined by transvaginal ultrasound. Approximately 70% were Caucasian, 25% were Black, and 5% were Asian, Native American, Pacific Islander, or Other. Seven percent (7%) of all subjects were of Hispanic origin. Women using hormonal contraception were excluded from the trials.
The rates of discontinuation due to adverse events during the two clinical trials were comparable between LYSTEDA and placebo. In the 3-cycle study, the rate in the 3900 mg LYSTEDA dose group was 0.8% as compared to 1.4% in the placebo group. In the 6-cycle study, the rate in the LYSTEDA group was 2.4% as compared to 4.1% in the placebo group. Across the studies, the combined exposure to 3900 mg/day LYSTEDA was 947 cycles and the average duration of use was 3.4 days per cycle.
A list of adverse events occurring in ≥ 5% of subjects and more frequently in LYSTEDA treated subjects receiving 3900 mg/day compared to placebo is provided in Table 2.

Postmarketing Experience
Long-term Studies
Long-term safety of LYSTEDA was studied in two open-label studies. In one study, subjects with physician-diagnosed heavy menstrual bleeding (not using the alkaline hematin methodology) were treated with 3900 mg/day for up to 5 days during each menstrual period for up to 27 menstrual cycles. A total of 781 subjects were enrolled and 239 completed the study through 27 menstrual cycles. A total of 12.4% of the subjects withdrew due to adverse events. Women using hormonal contraception were excluded from the study. The total exposure in this study to 3900 mg/day LYSTEDA was 10,213 cycles. The average duration of LYSTEDA use was 2.9 days per cycle.
A long-term open-label extension study of subjects from the two short-term efficacy studies was also conducted in which subjects were treated with 3900 mg/day for up to 5 days during each menstrual period for up to 9 menstrual cycles. A total of 288 subjects were enrolled and 196 subjects completed the study through 9 menstrual cycles. A total of 2.1% of the subjects withdrew due to adverse events. The total exposure to 3900 mg/day LYSTEDA in this study was 1,956 cycles. The average duration of LYSTEDA use was 3.5 days per cycle.
The types and severity of adverse events in these two long-term open-label trials were similar to those observed in the double-blind, placebo-controlled studies although the percentage of subjects reporting them was greater in the 27-month study, most likely because of the longer study duration.
A case of severe allergic reaction to LYSTEDA was reported in the extension trial, involving a subject on her fourth cycle of treatment, who experienced dyspnea, tightening of her throat, and facial flushing that required emergency medical treatment.
Drug Interactions
7.1 Hormonal Contraceptives Because LYSTEDA is antifibrinolytic, concomitant use of hormonal contraception and LYSTEDA may further exacerbate the increased thrombotic risk associated with combination hormonal contraceptives. For this reason, concomitant use of LYSTEDA with combination hormonal contraceptives is contraindicated [see CONTRAINDICATIONS (4) and WARNINGS AND PRECAUTIONS (5.1)].
7.2 Tissue Plasminogen Activators Concomitant therapy with tissue plasminogen activators may decrease the efficacy of both LYSTEDA and tissue plasminogen activators. Therefore, exercise caution if a woman taking LYSTEDA therapy requires tissue plasminogen activators.
7.3 Factor IX Complex Concentrates or Anti-Inhibitor Coagulant Concentrates LYSTEDA is not recommended for women taking either Factor IX complex concentrates or anti-inhibitor coagulant concentrates because the risk of thrombosis may be increased [see WARNINGS AND PRECAUTIONS (5.1) and CLINICAL PHARMACOLOGY (12.3)].
7.4 ALL-TRANS RETINOIC ACID (ORAL TRETINOIN) Exercise caution when prescribing LYSTEDA to women with acute promyelocytic leukemia taking all-trans retinoic acid for remission induction because of possible exacerbation of the procoagulant effect of all-trans retinoic acid [see WARNINGS AND PRECAUTIONS (5.1) and CLINICAL PHARMACOLOGY (12.3)].
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B LYSTEDA is not indicated for use in pregnant women. Reproduction studies have been performed in mice, rats and rabbits and have revealed no evidence of impaired fertility or harm to the fetus due to tranexamic acid. However, tranexamic acid is known to cross the placenta and appears in cord blood at concentrations approximately equal to the maternal concentration. There are no adequate and well-controlled studies in pregnant women [see NONCLINICAL TOXICOLOGY (13.1)].
An embryo-fetal developmental toxicity study in rats and a perinatal developmental toxicity study in rats were conducted using tranexamic acid. No adverse effects were observed in either study at doses up to 4 times the recommended human oral dose of 3900 mg/day based on mg/m2 (actual animal dose 1500 mg/kg/day).
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tranexamic acid (oral) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tranexamic acid (oral) during labor and delivery.
Nursing Mothers
Tranexamic acid is present in the mother's milk at a concentration of about one hundredth of the corresponding serum concentration. LYSTEDA should be used during lactation only if clearly needed.
Pediatric Use
LYSTEDA is indicated for women of reproductive age and is not intended for use in premenarcheal girls.
Based on a pharmacokinetic study in 20 adolescent females, 12 to 16 years of age, no dose adjustment is needed in the adolescent population.
Geriatic Use
LYSTEDA is indicated for women of reproductive age and is not intended for use by postmenopausal women.
Gender
There is no FDA guidance on the use of Tranexamic acid (oral) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tranexamic acid (oral) with respect to specific racial populations.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of LYSTEDA has not been studied. Because tranexamic acid is primarily eliminated via the kidneys by glomerular filtration with more than 95% excreted as unchanged in urine, dosage adjustment in patient with renal impairment is needed [see DOSAGE AND ADMINISTRATION (2.2) and CLINICAL PHARMACOLOGY (12.3)].
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of LYSTEDA has not been studied. Because only a small fraction of the drug is metabolized, dosage adjustment in patients with hepatic impairment is not needed [see CLINICAL PHARMACOLOGY (12.3)].
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tranexamic acid (oral) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tranexamic acid (oral) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Tranexamic acid (oral) Administration in the drug label.
Monitoring
There is limited information regarding Tranexamic acid (oral) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Tranexamic acid (oral) and IV administrations.
Overdosage
There are no known cases of intentional overdose with LYSTEDA and no subjects in the clinical program took more than 2 times the prescribed amount of LYSTEDA in a 24-hour period (>7800 mg/day). However, cases of overdose of tranexamic acid have been reported. Based on these reports, symptoms of overdose may include gastrointestinal (nausea, vomiting, diarrhea); hypotensive (e.g., orthostatic symptoms); thromboembolic (arterial, venous, embolic); visual impairment; mental status changes; myoclonus; or rash. No specific information is available on the treatment of overdose with LYSTEDA. In the event of overdose, employ the usual supportive measures (e.g., clinical monitoring and supportive therapy) as dictated by the patient's clinical status.
Pharmacology
| |
| |
Tranexamic acid (oral)
| |
| Systematic (IUPAC) name | |
| trans-4-(aminomethyl)cyclohexanecarboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | B02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 157.21 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 34% |
| Metabolism | ? |
| Half life | 3.1 h |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B |
| Legal status |
P (UK) |
| Routes | Injection and oral |
Mechanism of Action
There is limited information regarding Tranexamic acid (oral) Mechanism of Action in the drug label.
Structure
LYSTEDA is an antifibrinolytic drug. The chemical name is trans-4-aminomethyl-cyclohexanecarboxylic acid. The structural formula is:
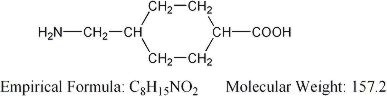
Tranexamic acid is a white crystalline powder. It is freely soluble in water and in glacial acetic acid and is very slightly soluble in ethanol and practically insoluble in ether. The molecular formula is C8H15N02 and the molecular weight is 157.2.
Tranexamic acid tablets are provided as white oval-shaped tablets and are not scored. Each tablet is debossed with the marking "FP650." The active ingredient in each tablet is 650 mg tranexamic acid. The inactive ingredients contained in each tablet are: microcrystalline cellulose, colloidal silicon dioxide, pregelatinized corn starch, povidone, hypromellose, stearic acid, and magnesium stearate.
Pharmacodynamics
There is limited information regarding Tranexamic acid (oral) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Tranexamic acid (oral) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Tranexamic acid (oral) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Tranexamic acid (oral) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Tranexamic acid (oral) How Supplied in the drug label.
Storage
There is limited information regarding Tranexamic acid (oral) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Tranexamic acid (oral) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tranexamic acid (oral) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Tranexamic acid (oral) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Tranexamic acid (oral) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Tranexamic acid (oral) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Tranexamic acid (oral) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.