Tranexamic acid (injection): Difference between revisions
No edit summary |
No edit summary |
||
| Line 10: | Line 10: | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult=====Indication==== | |fdaLIADAdult=====Indication==== | ||
* | *Tranexamic acid Injection is indicated in patients with hemophilia for short-term use (two to eight days) to reduce or prevent [[hemorrhage]] and reduce the need for replacement therapy during and following tooth extraction. | ||
====Dosage==== | ====Dosage==== | ||
*Immediately before tooth extraction in patients with hemophilia, administer 10 mg per kg body weight of | *Immediately before tooth extraction in patients with hemophilia, administer 10 mg per kg body weight of Tranexamic acid intravenously together with replacement therapy. | ||
*Following tooth extraction, intravenous therapy, at a dose of 10 mg per kg body weight three to four times daily, may be used for 2 to 8 days. | *Following tooth extraction, intravenous therapy, at a dose of 10 mg per kg body weight three to four times daily, may be used for 2 to 8 days. | ||
*Note: For patients with moderate to severe impaired [[renal function]], the following dosages are recommended: | *Note: For patients with moderate to severe impaired [[renal function]], the following dosages are recommended: | ||
| Line 19: | Line 19: | ||
[[File:Tranexamic acid1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | [[File:Tranexamic acid1.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | ||
For intravenous infusion, | For intravenous infusion, Tranexamic acid Injection may be mixed with most solutions for infusion such as electrolyte solutions, carbohydrate solutions, amino acid solutions, and Dextran solutions. [[Heparin]] may be added to Tranexamic acid Injection. Tranexamic acid Injection should NOT be mixed with blood. The drug is a synthetic amino acid, and should NOT be mixed with solutions containing [[penicillin]]. | ||
====Single-dose vials and ampules==== | ====Single-dose vials and ampules==== | ||
*Discard | *Discard Tranexamic acid vial or ampule and any remaining portion in the vial/ampule after single use. | ||
*The diluted mixture may be stored for up to 4 hours at room temperature prior to patient administration. | *The diluted mixture may be stored for up to 4 hours at room temperature prior to patient administration. | ||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tranexamic acid in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tranexamic acid in adult patients. | ||
|offLabelAdultNoGuideSupport=* Dental surgical procedure - Hemophilia - Hemorrhage. <ref name="pmid2963908">{{cite journal| author=Sindet-Pedersen S, Stenbjerg S, Ingerslev J| title=Control of gingival hemorrhage in hemophilic patients by inhibition of fibrinolysis with tranexamic acid. | journal=J Periodontal Res | year= 1988 | volume= 23 | issue= 1 | pages= 72-4 | pmid=2963908 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2963908 }} </ref> | |offLabelAdultNoGuideSupport=* Dental surgical procedure - Hemophilia - Hemorrhage. <ref name="pmid2963908">{{cite journal| author=Sindet-Pedersen S, Stenbjerg S, Ingerslev J| title=Control of gingival hemorrhage in hemophilic patients by inhibition of fibrinolysis with tranexamic acid. | journal=J Periodontal Res | year= 1988 | volume= 23 | issue= 1 | pages= 72-4 | pmid=2963908 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=2963908 }} </ref> | ||
|fdaLIADPed=The drug has had limited use in pediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for adults can be used for pediatric patients needing | |fdaLIADPed=The drug has had limited use in pediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for adults can be used for pediatric patients needing Tranexamic acid therapy. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Tranexamic acid in pediatric patients. | ||
|contraindications= | |contraindications=Tranexamic acid Injection is contraindicated: | ||
* In patients with acquired defective color vision, since this prohibits measuring one endpoint that should be followed as a measure of toxicity. | * In patients with acquired defective color vision, since this prohibits measuring one endpoint that should be followed as a measure of toxicity. | ||
* In patients with [[subarachnoid hemorrhage]]. Anecdotal experience indicates that [[cerebral edema]] and [[cerebral infarction]] may be caused by | * In patients with [[subarachnoid hemorrhage]]. Anecdotal experience indicates that [[cerebral edema]] and [[cerebral infarction]] may be caused by Tranexamic acid in such patients. | ||
* In patients with active intravascular clotting. | * In patients with active intravascular clotting. | ||
* In patients with [[hypersensitivity]] to tranexamic acid or any of the ingredients. | * In patients with [[hypersensitivity]] to tranexamic acid or any of the ingredients. | ||
| Line 48: | Line 48: | ||
===PRECAUTIONS=== | ===PRECAUTIONS=== | ||
====General==== | ====General==== | ||
* The dose of | * The dose of Tranexamic acid Injection should be reduced in patients with [[renal insufficiency]] because of the risk of accumulation. | ||
* Ureteral obstruction due to clot formation in patients with upper [[urinary tract bleeding]] has been reported in patients treated with | * Ureteral obstruction due to clot formation in patients with upper [[urinary tract bleeding]] has been reported in patients treated with Tranexamic acid. | ||
* Venous and arterial thrombosis or [[thromboembolism]] has been reported in patients treated with | * Venous and arterial thrombosis or [[thromboembolism]] has been reported in patients treated with Tranexamic acid. In addition, cases of central retinal artery and central retinal vein obstruction have been reported. | ||
* Patients with a previous history of thromboembolic disease may be at increased risk for venous or arterial thrombosis. | * Patients with a previous history of thromboembolic disease may be at increased risk for venous or arterial thrombosis. | ||
* | * Tranexamic acid should not be administered concomitantly with Factor IX Complex concentrates or Anti-inhibitor Coagulant concentrates, as the risk of thrombosis may be increased. | ||
* Patients with disseminated [[intravascular coagulation]] (DIC), who require treatment with | * Patients with disseminated [[intravascular coagulation]] (DIC), who require treatment with Tranexamic acid, must be under strict supervision of a physician experienced in treating this disorder. | ||
* Tranexamic acid may cause [[dizziness]] and therefore may influence the ability to drive or use machines. | * Tranexamic acid may cause [[dizziness]] and therefore may influence the ability to drive or use machines. | ||
| Line 64: | Line 64: | ||
|postmarketing=====Worldwide Postmarketing Reports==== | |postmarketing=====Worldwide Postmarketing Reports==== | ||
Thromboembolic events (e.g., [[deep vein thrombosis]], [[pulmonary embolism]], [[cerebral thrombosis]], acute renal cortical necrosis, and central retinal artery and vein obstruction) have been rarely reported in patients receiving tranexamic acid for indications other than [[hemorrhage]] prevention in patients with [[hemophilia]]. [[Convulsion]], chromatopsia, and [[visual impairment]] have also been reported. However, due to the spontaneous nature of the reporting of medical events and the lack of controls, the actual incidence and causal relationship of drug and event cannot be determined. | Thromboembolic events (e.g., [[deep vein thrombosis]], [[pulmonary embolism]], [[cerebral thrombosis]], acute renal cortical necrosis, and central retinal artery and vein obstruction) have been rarely reported in patients receiving tranexamic acid for indications other than [[hemorrhage]] prevention in patients with [[hemophilia]]. [[Convulsion]], chromatopsia, and [[visual impairment]] have also been reported. However, due to the spontaneous nature of the reporting of medical events and the lack of controls, the actual incidence and causal relationship of drug and event cannot be determined. | ||
|drugInteractions=No studies of interactions between | |drugInteractions=No studies of interactions between Tranexamic acid and other drugs have been conducted. | ||
|FDAPregCat=B | |FDAPregCat=B | ||
|useInPregnancyFDA=There are no adequate and well-controlled studies in pregnant women. However, tranexamic acid is known to pass the placenta and appears in cord blood at concentrations approximately equal to maternal concentration. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | |useInPregnancyFDA=There are no adequate and well-controlled studies in pregnant women. However, tranexamic acid is known to pass the placenta and appears in cord blood at concentrations approximately equal to maternal concentration. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. | ||
|useInLaborDelivery=See above under Pregnancy. | |useInLaborDelivery=See above under Pregnancy. | ||
|useInNursing=Tranexamic acid is present in the mother's milk at a concentration of about a hundredth of the corresponding serum levels. Caution should be exercised when | |useInNursing=Tranexamic acid is present in the mother's milk at a concentration of about a hundredth of the corresponding serum levels. Caution should be exercised when Tranexamic acid is administered to a nursing woman. | ||
|useInPed=The drug has had limited use in pediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for adults can be used for pediatric patients needing | |useInPed=The drug has had limited use in pediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for adults can be used for pediatric patients needing Tranexamic acid therapy. | ||
|useInGeri=Clinical studies of | |useInGeri=Clinical studies of Tranexamic acid did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | ||
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | ||
|useInRenalImpair=The dose of | |useInRenalImpair=The dose of Tranexamic acid Injection should be reduced in patients with renal insufficiency because of the risk of accumulation. | ||
|useInReproPotential=Reproduction studies performed in mice, rats, and rabbits have not revealed any evidence of impaired fertility or adverse effects on the fetus due to tranexamic acid. | |useInReproPotential=Reproduction studies performed in mice, rats, and rabbits have not revealed any evidence of impaired fertility or adverse effects on the fetus due to tranexamic acid. | ||
|administration=* Intravenous. | |administration=* Intravenous. | ||
|monitoring=* Monitor renal function in elderly patients because they are more likely to have decreased [[renal function]], care should be taken in dose selection. | |monitoring=* Monitor renal function in elderly patients because they are more likely to have decreased [[renal function]], care should be taken in dose selection. | ||
|IVCompat=There is limited information regarding IV Compatibility. | |IVCompat=There is limited information regarding IV Compatibility. | ||
|overdose=Cases of overdosage of | |overdose=Cases of overdosage of Tranexamic acid have been reported. Based on these reports, symptoms of overdosage may be gastrointestinal, e.g., [[nausea]], [[vomiting]], [[diarrhea]]; [[hypotensive]], e.g., orthostatic symptoms; thromboembolic, e.g., arterial, venous, embolic; neurologic, e.g., [[visual impairment]], [[convulsions]], [[headache]], [[Altered mental status|mental status changes]]; [[myoclonus]]; and [[rash]]. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| verifiedrevid = 477001216 | | verifiedrevid = 477001216 | ||
Revision as of 19:35, 27 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tranexamic acid (injection) is an antifibrinolytic agent that is FDA approved for the treatment of patients with hemophilia for short-term use (two to eight days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction. Common adverse reactions include abdominal pain, anemia, arthralgia, backache, cramps, musculoskeletal pain, headache, migraine, nasal sinus problem and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indication
- Tranexamic acid Injection is indicated in patients with hemophilia for short-term use (two to eight days) to reduce or prevent hemorrhage and reduce the need for replacement therapy during and following tooth extraction.
Dosage
- Immediately before tooth extraction in patients with hemophilia, administer 10 mg per kg body weight of Tranexamic acid intravenously together with replacement therapy.
- Following tooth extraction, intravenous therapy, at a dose of 10 mg per kg body weight three to four times daily, may be used for 2 to 8 days.
- Note: For patients with moderate to severe impaired renal function, the following dosages are recommended:
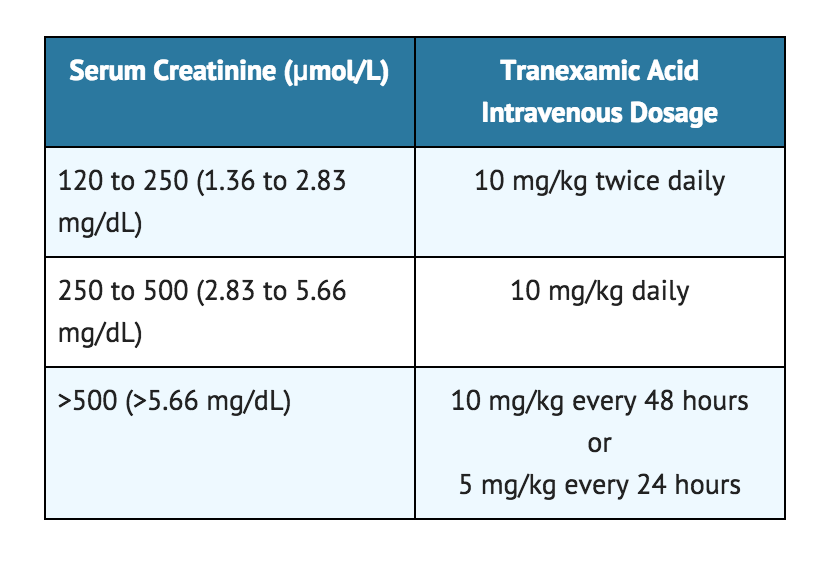
For intravenous infusion, Tranexamic acid Injection may be mixed with most solutions for infusion such as electrolyte solutions, carbohydrate solutions, amino acid solutions, and Dextran solutions. Heparin may be added to Tranexamic acid Injection. Tranexamic acid Injection should NOT be mixed with blood. The drug is a synthetic amino acid, and should NOT be mixed with solutions containing penicillin.
Single-dose vials and ampules
- Discard Tranexamic acid vial or ampule and any remaining portion in the vial/ampule after single use.
- The diluted mixture may be stored for up to 4 hours at room temperature prior to patient administration.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tranexamic acid in adult patients.
Non–Guideline-Supported Use
- Dental surgical procedure - Hemophilia - Hemorrhage. [1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The drug has had limited use in pediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for adults can be used for pediatric patients needing Tranexamic acid therapy.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Tranexamic acid in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tranexamic acid in pediatric patients.
Contraindications
Tranexamic acid Injection is contraindicated:
- In patients with acquired defective color vision, since this prohibits measuring one endpoint that should be followed as a measure of toxicity.
- In patients with subarachnoid hemorrhage. Anecdotal experience indicates that cerebral edema and cerebral infarction may be caused by Tranexamic acid in such patients.
- In patients with active intravascular clotting.
- In patients with hypersensitivity to tranexamic acid or any of the ingredients.
Warnings
Focal areas of retinal degeneration have developed in cats, dogs, and rats following oral or intravenous tranexamic acid at doses between 250 to 1600 mg/kg/day (6 to 40 times the recommended usual human dose) from 6 days to 1 year. The incidence of such lesions has varied from 25% to 100% of animals treated and was dose-related. At lower doses, some lesions have appeared to be reversible.
Limited data in cats and rabbits showed retinal changes in some animals with doses as low as 126 mg/kg/day (only about 3 times the recommended human dose) administered for several days to two weeks.
No retinal changes have been reported or noted in eye examinations in patients treated with tranexamic acid for weeks to months in clinical trials.
However, visual abnormalities, often poorly characterized, represent the most frequently reported postmarketing adverse reaction in Sweden. For patients who are to be treated continually for longer than several days, an ophthalmological examination, including visual acuity, color vision, eye-ground, and visual fields, is advised, before commencing and at regular intervals during the course of treatment. Tranexamic acid should be discontinued if changes in examination results are found.
Convulsions have been reported in association with tranexamic acid treatment, particularly in patients receiving tranexamic acid during cardiovascular surgery and in patients inadvertently given tranexamic acid into the neuraxial system.
PRECAUTIONS
General
- The dose of Tranexamic acid Injection should be reduced in patients with renal insufficiency because of the risk of accumulation.
- Ureteral obstruction due to clot formation in patients with upper urinary tract bleeding has been reported in patients treated with Tranexamic acid.
- Venous and arterial thrombosis or thromboembolism has been reported in patients treated with Tranexamic acid. In addition, cases of central retinal artery and central retinal vein obstruction have been reported.
- Patients with a previous history of thromboembolic disease may be at increased risk for venous or arterial thrombosis.
- Tranexamic acid should not be administered concomitantly with Factor IX Complex concentrates or Anti-inhibitor Coagulant concentrates, as the risk of thrombosis may be increased.
- Patients with disseminated intravascular coagulation (DIC), who require treatment with Tranexamic acid, must be under strict supervision of a physician experienced in treating this disorder.
- Tranexamic acid may cause dizziness and therefore may influence the ability to drive or use machines.
Adverse Reactions
Clinical Trials Experience
Gastrointestinal disturbances (nausea, vomiting, diarrhea) may occur but disappear when the dosage is reduced. Allergic dermatitis, giddiness, and hypotension have been reported occasionally. Hypotension has been observed when intravenous injection is too rapid. To avoid this response, the solution should not be injected more rapidly than 1 mL per minute.
Postmarketing Experience
Worldwide Postmarketing Reports
Thromboembolic events (e.g., deep vein thrombosis, pulmonary embolism, cerebral thrombosis, acute renal cortical necrosis, and central retinal artery and vein obstruction) have been rarely reported in patients receiving tranexamic acid for indications other than hemorrhage prevention in patients with hemophilia. Convulsion, chromatopsia, and visual impairment have also been reported. However, due to the spontaneous nature of the reporting of medical events and the lack of controls, the actual incidence and causal relationship of drug and event cannot be determined.
Drug Interactions
No studies of interactions between Tranexamic acid and other drugs have been conducted.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
There are no adequate and well-controlled studies in pregnant women. However, tranexamic acid is known to pass the placenta and appears in cord blood at concentrations approximately equal to maternal concentration. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tranexamic acid (injection) in women who are pregnant.
Labor and Delivery
See above under Pregnancy.
Nursing Mothers
Tranexamic acid is present in the mother's milk at a concentration of about a hundredth of the corresponding serum levels. Caution should be exercised when Tranexamic acid is administered to a nursing woman.
Pediatric Use
The drug has had limited use in pediatric patients, principally in connection with tooth extraction. The limited data suggest that dosing instructions for adults can be used for pediatric patients needing Tranexamic acid therapy.
Geriatic Use
Clinical studies of Tranexamic acid did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Tranexamic acid (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tranexamic acid (injection) with respect to specific racial populations.
Renal Impairment
The dose of Tranexamic acid Injection should be reduced in patients with renal insufficiency because of the risk of accumulation.
Hepatic Impairment
There is no FDA guidance on the use of Tranexamic acid (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
Reproduction studies performed in mice, rats, and rabbits have not revealed any evidence of impaired fertility or adverse effects on the fetus due to tranexamic acid.
Immunocompromised Patients
There is no FDA guidance one the use of Tranexamic acid (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous.
Monitoring
- Monitor renal function in elderly patients because they are more likely to have decreased renal function, care should be taken in dose selection.
IV Compatibility
There is limited information regarding IV Compatibility.
Overdosage
Cases of overdosage of Tranexamic acid have been reported. Based on these reports, symptoms of overdosage may be gastrointestinal, e.g., nausea, vomiting, diarrhea; hypotensive, e.g., orthostatic symptoms; thromboembolic, e.g., arterial, venous, embolic; neurologic, e.g., visual impairment, convulsions, headache, mental status changes; myoclonus; and rash.
Pharmacology
| |
| |
Tranexamic acid (injection)
| |
| Systematic (IUPAC) name | |
| trans-4-(aminomethyl)cyclohexanecarboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | B02 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 157.21 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 34% |
| Metabolism | ? |
| Half life | 3.1 h |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B |
| Legal status |
P (UK) |
| Routes | Injection and oral |
Mechanism of Action
Tranexamic acid is a competitive inhibitor of plasminogen activation, and at much higher concentrations, a noncompetitive inhibitor of plasmin, i.e., actions similar to aminocaproic acid. Tranexamic acid is about 10 times more potent in vitro than aminocaproic acid.
Structure
Each mL of the sterile solution for intravenous injection contains 100 mg tranexamic acid and Water for Injection to 1 mL.
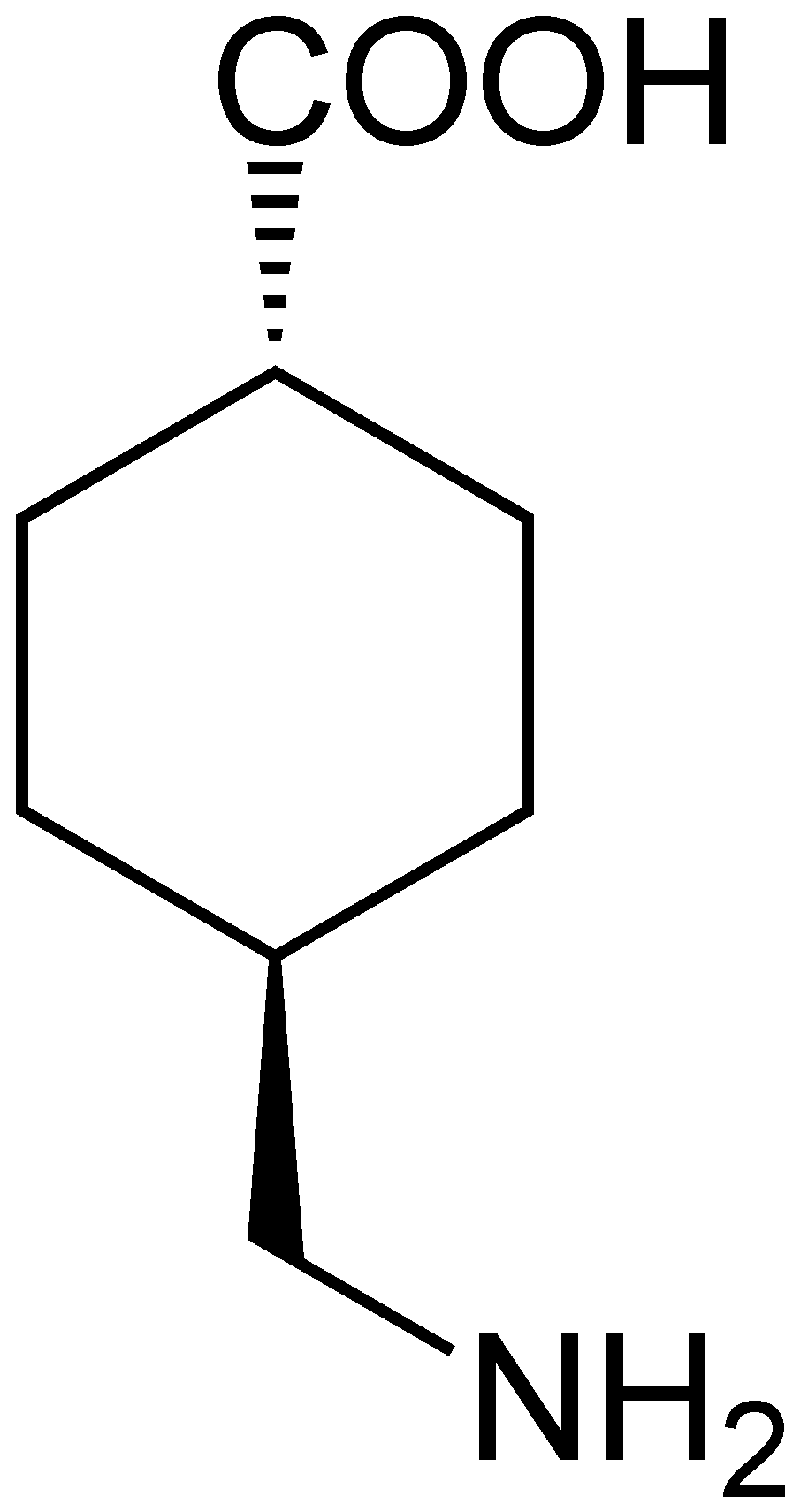
Chemical Name: trans-4-(aminomethyl)cyclohexanecarboxylic acid
Structural Formula:
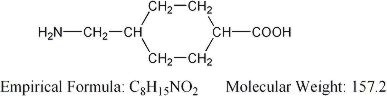
Tranexamic acid is a white crystalline powder. The aqueous solution for injection has a pH of 6.5 to 8.0.
Pharmacodynamics
There is limited information regarding pharmacodynamics.
Pharmacokinetics
Tranexamic acid binds more strongly than aminocaproic acid to both the strong and weak receptor sites of the plasminogen molecule in a ratio corresponding to the difference in potency between the compounds. Tranexamic acid in a concentration of 1 mg per mL does not aggregate platelets in vitro.
Tranexamic acid, in concentrations as low as 1 mg per mL, can prolong the thrombin time. However, tranexamic acid in concentrations up to 10 mg per mL in blood showed no influence on the platelet count, the coagulation time, or other coagulation factors in whole blood or citrated blood from normal subjects.
The plasma protein binding of tranexamic acid is about 3% at therapeutic plasma levels and seems to be fully accounted for by its binding to plasminogen. Tranexamic acid does not bind to serum albumin.
After an intravenous dose of 1 g, the plasma concentration time curve shows a triexponential decay with a half-life of about 2 hours for the terminal elimination phase. The initial volume of distribution is about 9 to 12 liters. Urinary excretion is the main route of elimination via glomerular filtration. Overall renal clearance is equal to overall plasma clearance (110 to 116 mL/min), and more than 95% of the dose is excreted in the urine as unchanged drug. Excretion of tranexamic acid is about 90% at 24 hours after intravenous administration of 10 mg per kg body weight.
An antifibrinolytic concentration of tranexamic acid remains in different tissues for about 17 hours, and in the serum, up to seven or eight hours.
Tranexamic acid passes through the placenta. The concentration in cord blood after an intravenous injection of 10 mg per kg to pregnant women is about 30 mg per liter, as high as in the maternal blood. Tranexamic acid diffuses rapidly into joint fluid and the synovial membrane. In the joint fluid, the same concentration is obtained as in the serum. The biological half-life of tranexamic acid in the joint fluid is about three hours.
The concentration of tranexamic acid in a number of other tissues is lower than in blood. In breast milk, the concentration is about one hundredth of the serum peak concentration. Tranexamic acid concentration in cerebrospinal fluid is about one tenth of that of the plasma. The drug passes into the aqueous humor, the concentration being about one tenth of the plasma concentration.
Tranexamic acid has been detected in semen where it inhibits fibrinolytic activity but does not influence sperm migration.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility An increased incidence of leukemia in male mice receiving tranexamic acid in food at a concentration of 4.8% (equivalent to doses as high as 5 g/kg/day) may have been related to treatment. Female mice were not included in this experiment.
Hyperplasia of the biliary tract and cholangioma and adenocarcinoma of the intrahepatic biliary system have been reported in one strain of rats after dietary administration of doses exceeding the maximum tolerated dose for 22 months. Hyperplastic, but not neoplastic, lesions were reported at lower doses. Subsequent long-term dietary administration studies in a different strain of rat, each with an exposure level equal to the maximum level employed in the earlier experiment, have failed to show such hyperplastic / neoplastic changes in the liver. No mutagenic activity has been demonstrated in several in vitro and in vivo test systems.
Clinical Studies
There is limited information regarding clinical studies.
How Supplied
CYKLOKAPRON Injection 100 mg/mL NDC 0013-1114-10 10 × 10 mL single-dose ampules
CYKLOKAPRON Injection 100 mg/mL NDC 0013-1114-21 10 × 10 mL single-dose vials
Storage
Store at 25°C (77°F); excursions permitted to 15°–30°C (59°–86°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Tranexamic acid (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
fizer Injectables
Rx only
NDC 0013-1114-01
10 mL ampule Cyklokapron® tranexamic acid injection 1000 mg/10 mL (100 mg/mL) Solution for intravenous injection Single-Dose ONLY Discard any remaining portion after single use
PAA058409

NDC 0013-1114-01
Contains 1 Ampule 10 mL Cyklokapron® tranexamic acid injection
1000 mg/10 mL (100 mg/mL)
Solution for intravenous injection
Single-Dose ONLY Discard any remaining portion after single use
Rx only
Pfizer Injectables

NDC 0013-1114-10
Contains 10 of NDC 0013-1114-01
10 x 10 mL ampules
Cyklokapron®
tranexamic acid injection
1000 mg/10 mL (100 mg/mL) Solution for intravenous injection
Single-Dose ONLY
Discard any remaining
portion after single use
Rx only

NDC 0013-1114-20
10 mL Vial
Cyklokapron®
(tranexamic acid injection)
1000 mg/10 mL
(100 mg/mL) Rx only

NDC 0013-1114-20
Contains 1 Vial 10 mL
Cyklokapron® (tranexamic acid injection)
1000 mg/10 mL (100 mg/mL)
Solution for intravenous injection
Single-Dose ONLY Discard any remaining portion after single use
Rx only
Pfizer Injectables
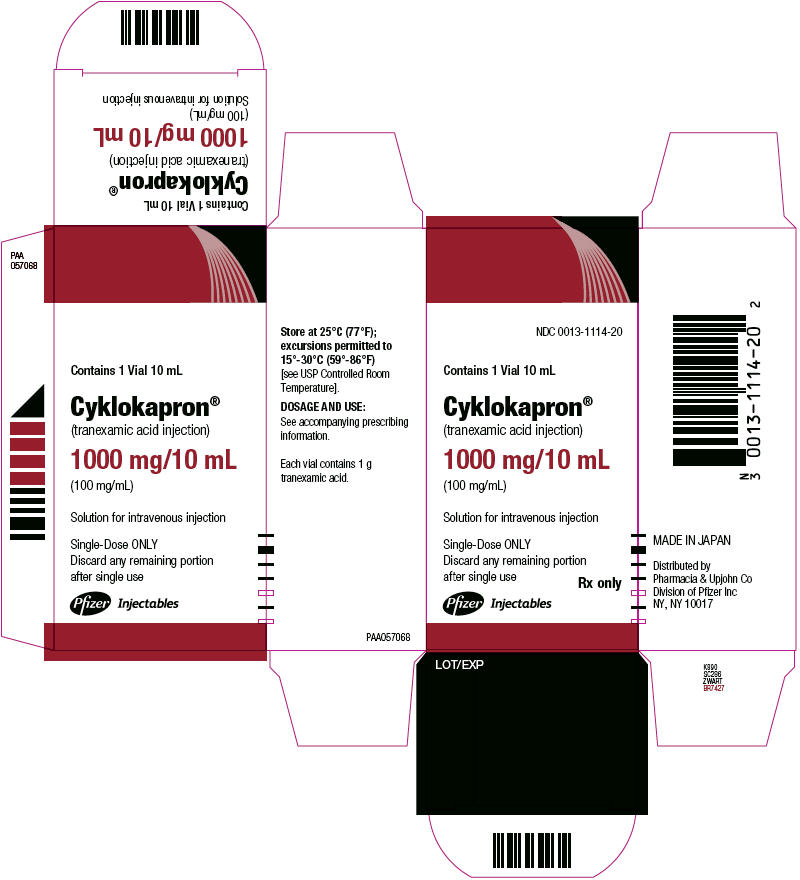
LOT/EXP
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F) [see USP Controlled Room Temperature].
DOSAGE AND USE: See accompanying prescribing information.
Each vial contains 1 g tranexamic acid.
MADE IN JAPAN
Distributed by Pharmacia & Upjohn Co Division of Pfizer Inc, NY, NY 10017
NDC 0013-1114-21 Contains 10 of NDC 0013-1114-20
10 x 10 mL Vials
Cyklokapron®
(tranexamic acid injection) 1000 mg/10 mL (100 mg/mL)
Solution for intravenous injection
Single-Dose ONLY Discard any remaining portion after single use
Rx only
PAA057067

Ingredients and Appearance
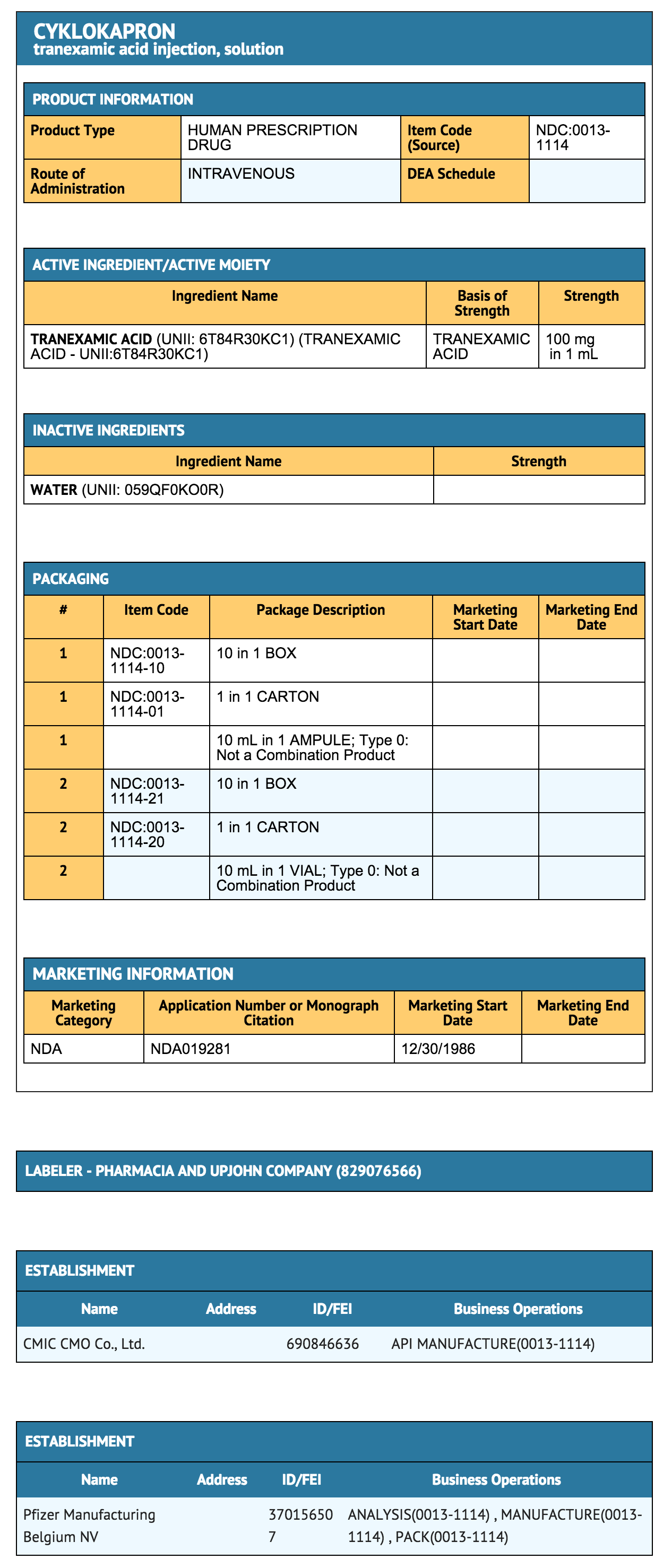
{{#ask: Label Page::Tranexamic acid (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding patient counseling information .
Precautions with Alcohol
Alcohol-Tranexamic acid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CYKLOKAPRON ®[2]
Look-Alike Drug Names
There is limited information regarding Tranexamic acid (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Sindet-Pedersen S, Stenbjerg S, Ingerslev J (1988). "Control of gingival hemorrhage in hemophilic patients by inhibition of fibrinolysis with tranexamic acid". J Periodontal Res. 23 (1): 72–4. PMID 2963908.
- ↑ "CYKLOKAPRON- tranexamic acid injection, solution".