Tasosartan: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 34: | Line 34: | ||
It was withdrawn from FDA review by the manufacturer after [[clinical trials#Phase III|phase III clinical trials]] showed [[elevated transaminases]] (a sign of possible [[hepatotoxicity|liver toxicity]]) in a significant number of participants given the drug.<ref>{{cite book |author=Atkinson AJ et al. |title=Principles of clinical pharmacology |publisher=Elsevier |location=Amsterdam |year=2007 |page=515 |isbn=0-12-369417-5}}</ref><ref>{{cite journal |author=Dina R, Jafari M |title=Angiotensin II-receptor antagonists: an overview |journal=Am J Health Syst Pharm |volume=57 |issue=13 |pages=1231–41 |date=July 2000 |pmid=10902066 |url=http://www.medscape.com/viewarticle/406895}}</ref> | It was withdrawn from FDA review by the manufacturer after [[clinical trials#Phase III|phase III clinical trials]] showed [[elevated transaminases]] (a sign of possible [[hepatotoxicity|liver toxicity]]) in a significant number of participants given the drug.<ref>{{cite book |author=Atkinson AJ et al. |title=Principles of clinical pharmacology |publisher=Elsevier |location=Amsterdam |year=2007 |page=515 |isbn=0-12-369417-5}}</ref><ref>{{cite journal |author=Dina R, Jafari M |title=Angiotensin II-receptor antagonists: an overview |journal=Am J Health Syst Pharm |volume=57 |issue=13 |pages=1231–41 |date=July 2000 |pmid=10902066 |url=http://www.medscape.com/viewarticle/406895}}</ref> | ||
==References== | ==References== | ||
Revision as of 20:19, 23 July 2014
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
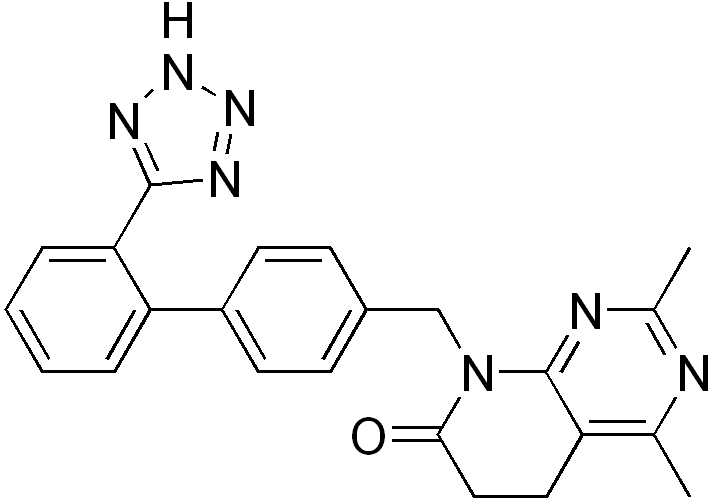
| Formula | C23H21N7O |
| Molar mass | 411.459 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Sheng Shi, M.D. [2]
Overview
Tasosartan is an angiotensin II receptor antagonist.
It was withdrawn from FDA review by the manufacturer after phase III clinical trials showed elevated transaminases (a sign of possible liver toxicity) in a significant number of participants given the drug.[1][2]
References
- ↑ Atkinson AJ; et al. (2007). Principles of clinical pharmacology. Amsterdam: Elsevier. p. 515. ISBN 0-12-369417-5.
- ↑ Dina R, Jafari M (July 2000). "Angiotensin II-receptor antagonists: an overview". Am J Health Syst Pharm. 57 (13): 1231–41. PMID 10902066.
Categories:
- Pages with script errors
- CS1 maint: Explicit use of et al.
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Tetrazoles
- Lactams
- Angiotensin II receptor antagonists
- Cardiovascular Drugs
- Drugs