Sandbox : anmol
|
Hyperparathyroidism Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sandbox : anmol On the Web |
|
American Roentgen Ray Society Images of Sandbox : anmol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Anmol Pitliya, M.B.B.S. M.D.[2]
Classification
| Classification of hyperparathyridism | |||
|---|---|---|---|
| Features | Primary hyperparathyroidism | Secondary hyperparathyroidism | Tertiary hyperparathyroidism |
| Pathology | Hyperfunction of parathyroid cells due to hyperplasia, adenoma or carcinoma. | Physiological stimulation of parathyroid in response to hypocalcaemia. | Following long term physiological stimulation leading to hyperplasia. |
| Cause | |||
| Associations | May be associated with multiple endocrine neoplasia. | Usually due to chronic renal failure or other causes of Vitamin D deficiency. | Seen in chronic renal failure. |
| Serum calcium | High | Low/Normal | High |
| Serum phosphate | Low/Normal | High | High |
| Management | Usually surgery if symptomatic. Cincacalcet can be considered in those not fit for surgery. | Treatment of underlying cause. | Usually cinacalcet or surgery in those that don't respond. |
Causes
Genetic causes
- HRPT2 gene mutations:[1]
- HRPT2 gene code for parafibromin protein.
- HRPT2 gene mutations are found in a type of familial hyperparathyroidism, hyperparathyroidism-jaw tumor (HPT-JT) syndrome.
- HRTP2 gene mutations increases risk of parathyroid carcinoma.
- Cyclin D1 gene (CCND1)/PRAD1 gene:[2][3]
- PRAD1 (parathyroid adenoma 1) is a protooncogene located on chromosome 11q13.
- Cyclin D1 gene translocation and oncogene action observerd in 8% of adenomas
- Cyclin D1 gene overexpression is pbserved in 20% to 40% of parathyroid adenomas
- MEN1 gene:[2][4]
- MEN1 is a tumor supressor gene on chronosome 11q13.
- Somatic loss of single MEN1 allele is observed in 25% to 40% of sporadic parathyroid adenomas.
Pathogenesis
Associated conditions
- Hypercalcemia
- Chronic renal failure
- Osteitis fibrous cystica
- Osteoporosis
- Osteomalacia
- Osteoarthritis
- Brown tumor
- Multiple endocrine neoplasia type 1, type 2A, and type 4
- Familial isolated hyperparathyroidism
- Neonatal severe hyperparathyroidism
- Familial hypocalciuric hypercalcemia
- Hyperparathyroid-jaw tumor syndrome
- Pancreatitis[5]
ECG
There are no CT scan findings associated with hyperparathyroidism. However, a CT scan may be helpful in the diagnosis of cardiac complications of hyperparathyroidism. Findings on ECG are due to hypercalcemia and includes:[6]
- ST segment - ST segment is short in patients with hyperparathyroidism when compared to normocalcemic patients. This represents a decrease in systolic interval.
- QRS complex - QRS complex has an increased amplitudein patients with hyperparathyroidism when compared to normocalcemic patients. This represents an increase in ventricular muscle mass.
- T wave - T wave is prolonged in patients with hyperparathyroidism when compared to normocalcemic patients.
X-ray
CT scan
MRI
Ultrasound
TC-99m Sestamibi Scintigraphy
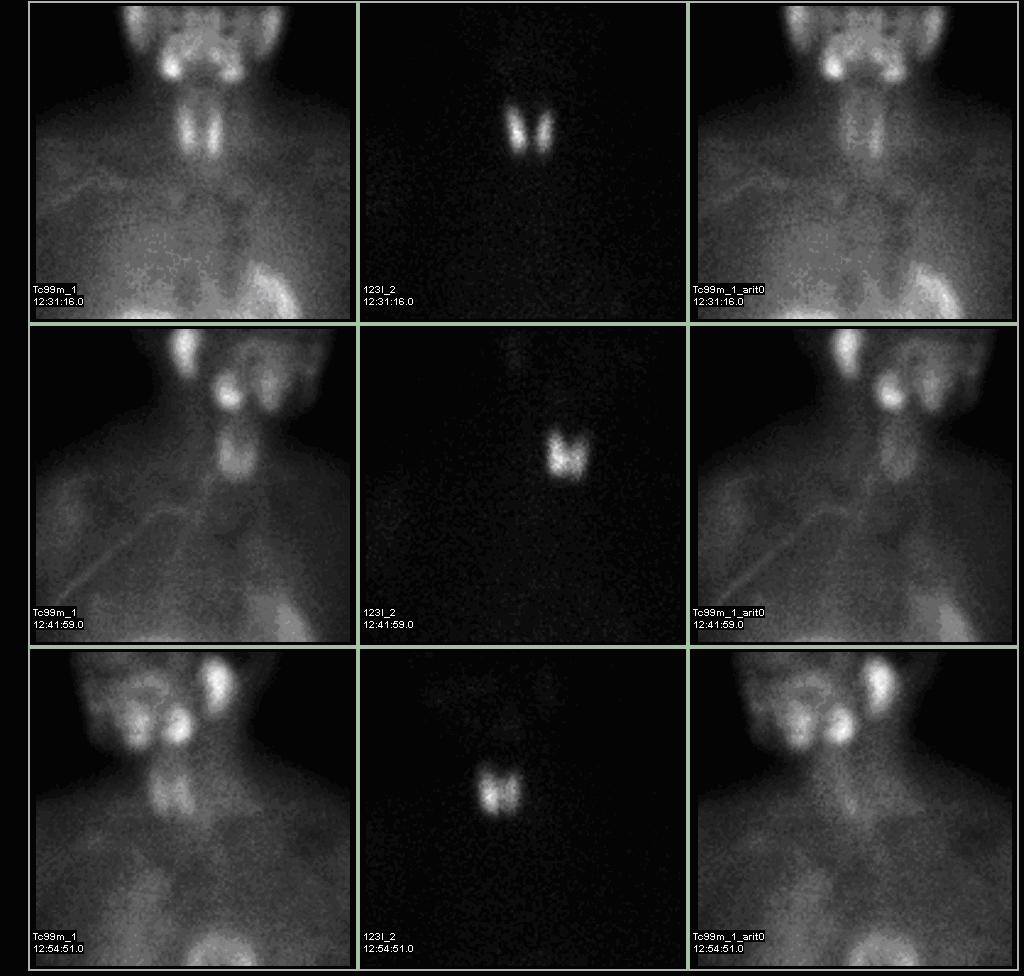
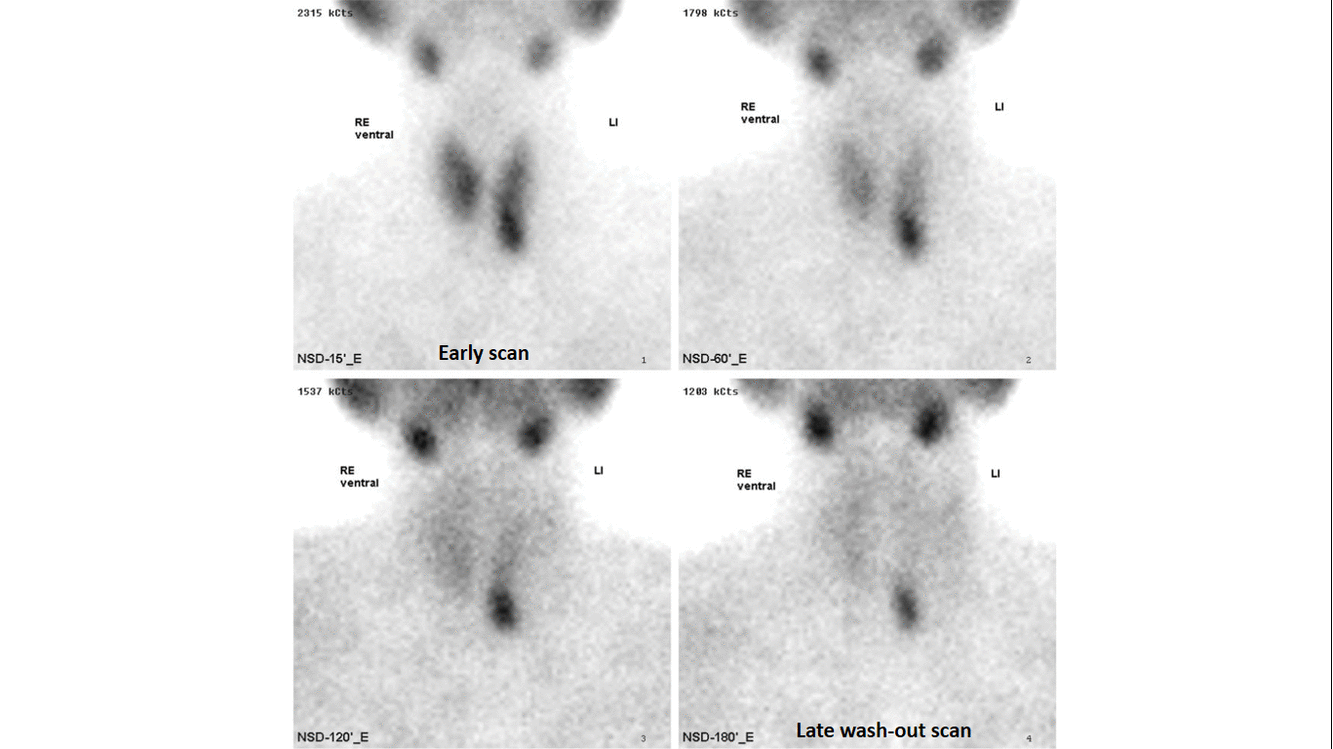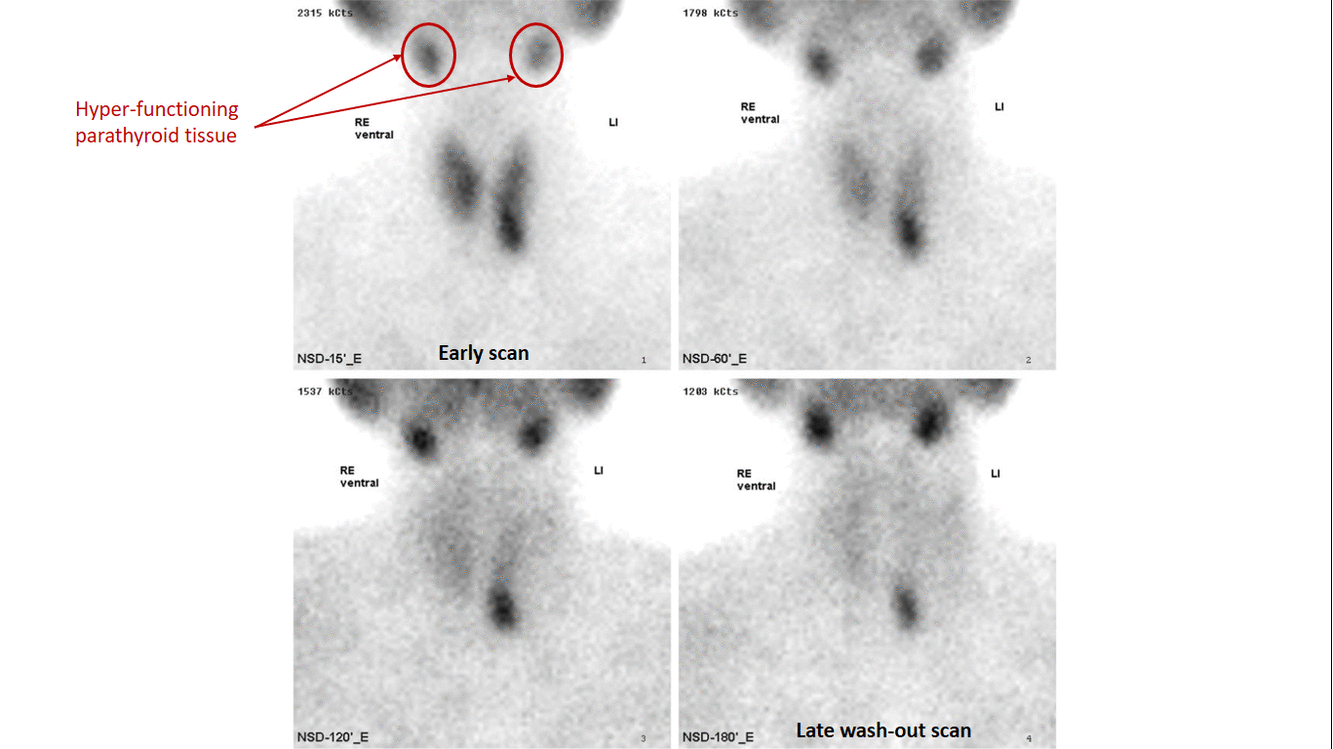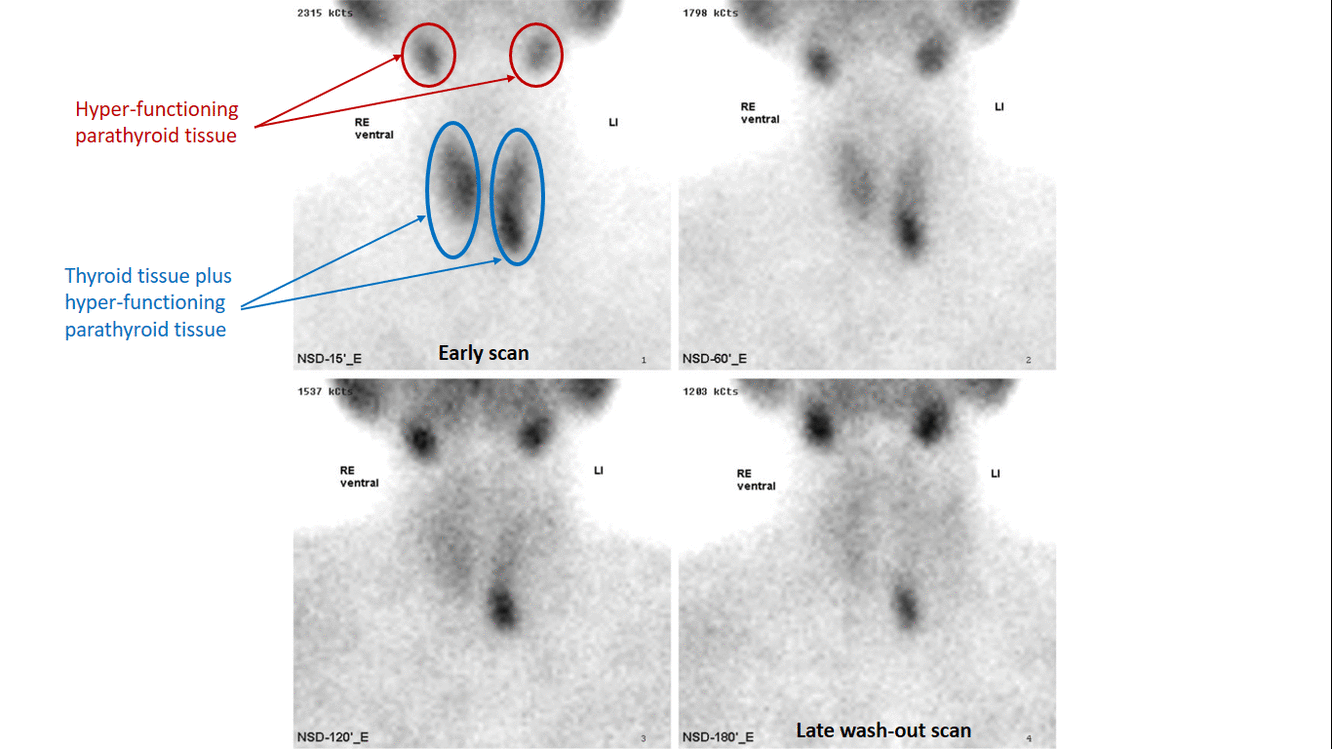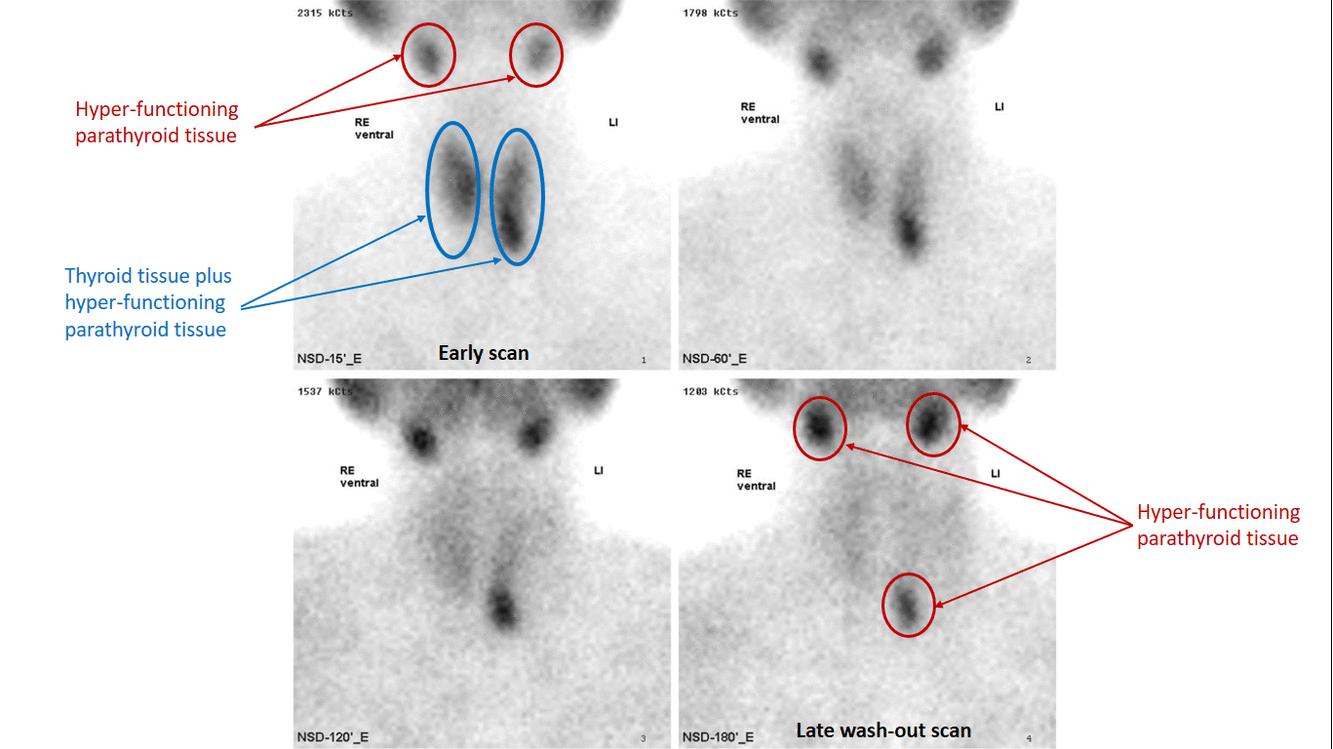
- Technetium-99m-methoxyisobutylisonitrile (99mTc-sestamibi or MIBI) scintigraphy is the most popular investigation for preoperative localization of hyper-functioning parathyroid glands.[7]
- Most of the sestamibi is retained in mitochondria of thyroid and abnormal parathyroid tissue and is a function of mitochondrial activity.[8]
- The basis of this "single-isotope, double-phase technique" is that sestamibi washes out of the thyroid more rapidly than from abnormal parathyroid tissue.[9]
- Multiple planar images are obtained, typically one shortly after injection of 99mTc-sestamibi and another after two hours to identify the foci of retained sestamibi showing hyper-functioning parathyroid tissue.
- As all parathyroid lesions does not retain sestamibi nor all thyroid tissue washes out quickly, subtraction imaging may be beneficial.[10]
- Subtraction technique uses dual contrast Tc-99m sestamibi along with iodine-123 or 99m-technicium pertechnetate which is taken by thyroid tissue only. Iodine-123/99m-technicium pertechnetate images of thyroid are later digitally subtracted from Tc-99m sestamibi images leading to visualization of parathyroid tissue only.[11]
- Presence of solid thyroid nodule is the most common cause of false positive results. Other causes of false positive results may include thyroid carcinoma, lymphoma, and lymphadenopathy.
- The sensitivity of sestamibi scintigraphy can be increased by using it concomitantly with neck ultrasound and/or SPECT. [12][13]
- The sensitivity of sestamibi scintigraphy is 80% - 90%.[14][15][16]
 |
 |
SPECT
- Single positron emission computed tomography may be used along with Tc-99m sestamibi scintigraphy for preoperative evaluation of hyper-functioning parathyroid gland.[17][18]
- Sestamibi-SPECT is also called pinhone-SPECT (P-SPECT). P-SPECT uses cone beam collimator in contrast to parallel-hole collimator used in SPECT. cone bean collimator possess more suitable geometric properties leading to high spatial resolution.[19][20]
- Using SPECT with sestamibi scintigraphy improves detection and localization of hyper-functioning parathyroid gland.[21][22]
- SPECT provides more precise result of sestamibi scitigraphy allowing surgeon to choose best route for surgical intervention.
- P-SPECT may detect glands not visible on planer images leading to increased sensitivity. It is very useful in case of uncertain result from conventional sestamibi scitigraphy.[23][24]
- P-SPECT also enables accurate interpretation sestamibi uptake in upper mediastinum leading to a higher specificity.
- In difficult cases, P-SPECT may also be adjuncted with subtraction Tc-99m sestamibi and I-123 scintigraphy or positron emission tomography.[25]
- P-SPECT is approximately 84% sensitive, 91% specific with positive predictive value of around 91% and negative predictive value of around 84%.[26]
- Fusion images of CT-MIBI-SPECT is superior to CT or MIBI-SPECT alone in preoperative localization of hyper-functioning parathyroid gland.[27]
PET
- 11C-methionine PET along with CT scan (MET-PET/CT) may be used for preoperative localization of hyper-functioning gland.[28][29]
- MET-PET/CT may be used as an complimentary imaging modality for localizing hyper-functioning parathyroid glands in patients with negative Tc-99m sestamibi scintigraphy/SPECT results.[30]
DXA
- Low bone mineral density (BMD) is caused by primary hyperparathyroidism. Distal forearm is affected most commonly.
- DXA of distal forearm should be done in all patients of primary hyperparathyroidism. Worst T-score of distal forearm is observed in patients with primary hyperparathyroidism.[31]
Other diagnostic studies
Intraoperative parathyroid hormone (IOPTH)
- Measurement of intraoperative parathyroid hormone (IOPTH) by using a modified sensitive assay (immunoradiometric assay) is beneficial for long term surgical outcomes.Post-surgical success is defined as postoperative normocalcemia.
- Patients with hyperparathyroidism due to lesion in a single gland shows a rapid decline of intact parathyroid hormone. The levels of intact parathyroid hormone reached to indetectable levels within hours of resection.[32]
- After resection of parathyroid adenoma, intact parathyroid hormone levels decrease by 85% is observed in first 15 minutes. This fall in parathyroid hormone levels is due to short half-life of parathyroid hormone.[33]
- The fall in parathyroid hormone level is significantly more after resection of parathyroid adenoma than after resection of parathyroid hyperplasia.
- A fall in level of parathyroid hormone 15 minutes after resection of hyper-functioning parathyroid glands may help differentiating sigle gland disease from multi gland disease.[34][35]
- IOPTH monitoring has a predictive accuracy of 97%. [36]
Technique for intraoperative parathyroid hormone (IOPTH) monitoring
- When the enlarged parathyroid gland is first visualized intraoperatively, the baseline sample should be obtained.[37]
- The baseline samples should never be obtained before induction of anesthesia. It is due to the fact that an increase in parathyroid hormone level may be observed after general anesthesia.
- After excision of enlarged gland, 2nd and 3rd samples are collected at 5 and 10 minutes respectively.
- Several criteria are used for predicting post-operative normocalcemia including:
- A decline in parathyroid hormone levels of ≥60% from baseline value at 15 minutes.
- A decline in parathyroid hormone levels of ≥50% from baseline value at 10 minutes.
Super Selective Venous Sampling
Selective arteriography
- Selective transarterial hypocalcemic stimulation is combined with nonselective venous sampling to perform selective arteriography.[38]
- Sodium citrate is injected to induce hypocalcemia. Simultaneous arteriography is performed.
- Samples are taken for superior vena cava at basaeline and timed intervals (20 sec, 40 sec, and 60 sec).
- An increase in the parathyroid hormone level to 1.4 times above the baseline or a clear blush observed on arteriography is considered as positive localization.
- Arterial stimulation venous sampling is performed simultaneously with arteriogram due to similarly high PPV.
Angiography
- Superselective arterial digital subtraction angiography (DSA) and superselective conventional angiography (CA) may be used for preoperative localization of hyper-functioning parathyroid glands in which noninvasive imaging modalities are negative or inconclusive.[39]
- Sensitivity of superselective digital subtraction angiography appears to be similar to conventional angiography.
- Superselective arterial digital subtraction angiography may be more sensitive than conventional angiography for preoperative localization of mediastinal hyper-functioning parathyroid glands.
References
- ↑ Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D; et al. (2003). "Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma". N Engl J Med. 349 (18): 1722–9. doi:10.1056/NEJMoa031237. PMID 14585940.
- ↑ 2.0 2.1 Westin G, Björklund P, Akerström G (2009). "Molecular genetics of parathyroid disease". World J Surg. 33 (11): 2224–33. doi:10.1007/s00268-009-0022-6. PMID 19373510.
- ↑ Hsi ED, Zukerberg LR, Yang WI, Arnold A (1996). "Cyclin D1/PRAD1 expression in parathyroid adenomas: an immunohistochemical study". J Clin Endocrinol Metab. 81 (5): 1736–9. doi:10.1210/jcem.81.5.8626826. PMID 8626826.
- ↑ Agarwal SK, Kester MB, Debelenko LV, Heppner C, Emmert-Buck MR, Skarulis MC; et al. (1997). "Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states". Hum Mol Genet. 6 (7): 1169–75. PMID 9215689.
- ↑ Bai HX, Giefer M, Patel M, Orabi AI, Husain SZ (2012). "The association of primary hyperparathyroidism with pancreatitis". J. Clin. Gastroenterol. 46 (8): 656–61. doi:10.1097/MCG.0b013e31825c446c. PMC 4428665. PMID 22874807.
- ↑ Lind L, Ljunghall S (1994). "Serum calcium and the ECG in patients with primary hyperparathyroidism". J Electrocardiol. 27 (2): 99–103. PMID 8201301.
- ↑ Palestro CJ, Tomas MB, Tronco GG (2005). "Radionuclide imaging of the parathyroid glands". Semin Nucl Med. 35 (4): 266–76. doi:10.1053/j.semnuclmed.2005.06.001. PMID 16150247.
- ↑ Hetrakul N, Civelek AC, Stagg CA, Udelsman R (2001). "In vitro accumulation of technetium-99m-sestamibi in human parathyroid mitochondria". Surgery. 130 (6): 1011–8. doi:10.1067/msy.2001.118371. PMID 11742331.
- ↑ Taillefer R, Boucher Y, Potvin C, Lambert R (1992). "Detection and localization of parathyroid adenomas in patients with hyperparathyroidism using a single radionuclide imaging procedure with technetium-99m-sestamibi (double-phase study)". J Nucl Med. 33 (10): 1801–7. PMID 1328564.
- ↑ Thulé P, Thakore K, Vansant J, McGarity W, Weber C, Phillips LS (1994). "Preoperative localization of parathyroid tissue with technetium-99m sestamibi 123I subtraction scanning". J Clin Endocrinol Metab. 78 (1): 77–82. doi:10.1210/jcem.78.1.8288719. PMID 8288719.
- ↑ Ryhänen EM, Schildt J, Heiskanen I, Väisänen M, Ahonen A, Löyttyniemi E; et al. (2015). "(99m)Technetium Sestamibi-(123)Iodine Scintigraphy Is More Accurate Than (99m)Technetium Sestamibi Alone before Surgery for Primary Hyperparathyroidism". Int J Mol Imaging. 2015: 391625. doi:10.1155/2015/391625. PMC 4333274. PMID 25722888.
- ↑ Eslamy HK, Ziessman HA (2008). "Parathyroid scintigraphy in patients with primary hyperparathyroidism: 99mTc sestamibi SPECT and SPECT/CT". Radiographics. 28 (5): 1461–76. doi:10.1148/rg.285075055. PMID 18794320.
- ↑ Haber RS, Kim CK, Inabnet WB (2002). "Ultrasonography for preoperative localization of enlarged parathyroid glands in primary hyperparathyroidism: comparison with (99m)technetium sestamibi scintigraphy". Clin Endocrinol (Oxf). 57 (2): 241–9. PMID 12153604.
- ↑ Chapuis Y, Fulla Y, Bonnichon P, Tarla E, Abboud B, Pitre J, Richard B (1996). "Values of ultrasonography, sestamibi scintigraphy, and intraoperative measurement of 1-84 PTH for unilateral neck exploration of primary hyperparathyroidism". World J Surg. 20 (7): 835–9, discussion 839–40. PMID 8678959.
- ↑ Prasannan S, Davies G, Bochner M, Kollias J, Malycha P (2007). "Minimally invasive parathyroidectomy using surgeon-performed ultrasound and sestamibi". ANZ J Surg. 77 (9): 774–7. doi:10.1111/j.1445-2197.2007.04227.x. PMID 17685957.
- ↑ Gómez-Ramírez J, Sancho-Insenser JJ, Pereira JA, Jimeno J, Munné A, Sitges-Serra A (2010). "Impact of thyroid nodular disease on 99mTc-sestamibi scintigraphy in patients with primary hyperparathyroidism". Langenbecks Arch Surg. 395 (7): 929–33. doi:10.1007/s00423-010-0680-8. PMID 20625763.
- ↑ Billotey C, Sarfati E, Aurengo A, Duet M, Mündler O, Toubert ME; et al. (1996). "Advantages of SPECT in technetium-99m-sestamibi parathyroid scintigraphy". J Nucl Med. 37 (11): 1773–8. PMID 8917173.
- ↑ Civelek AC, Ozalp E, Donovan P, Udelsman R (2002). "Prospective evaluation of delayed technetium-99m sestamibi SPECT scintigraphy for preoperative localization of primary hyperparathyroidism". Surgery. 131 (2): 149–57. PMID 11854692.
- ↑ Strand SE, Ivanovic M, Erlandsson K, Franceschi D, Button T, Sjögren K; et al. (1994). "Small animal imaging with pinhole single-photon emission computed tomography". Cancer. 73 (3 Suppl): 981–4. PMID 8306288.
- ↑ Jaszczak RJ, Li J, Wang H, Zalutsky MR, Coleman RE (1994). "Pinhole collimation for ultra-high-resolution, small-field-of-view SPECT". Phys Med Biol. 39 (3): 425–37. PMID 15551591.
- ↑ Schachter PP, Issa N, Shimonov M, Czerniak A, Lorberboym M (2004). "Early, postinjection MIBI-SPECT as the only preoperative localizing study for minimally invasive parathyroidectomy". Arch Surg. 139 (4): 433–7. doi:10.1001/archsurg.139.4.433. PMID 15078713.
- ↑ Perez-Monte JE, Brown ML, Shah AN, Ranger NT, Watson CG, Carty SE; et al. (1996). "Parathyroid adenomas: accurate detection and localization with Tc-99m sestamibi SPECT". Radiology. 201 (1): 85–91. doi:10.1148/radiology.201.1.8816526. PMID 8816526.
- ↑ Spanu A, Falchi A, Manca A, Marongiu P, Cossu A, Pisu N; et al. (2004). "The usefulness of neck pinhole SPECT as a complementary tool to planar scintigraphy in primary and secondary hyperparathyroidism". J Nucl Med. 45 (1): 40–8. PMID 14734671.
- ↑ Carlier T, Oudoux A, Mirallié E, Seret A, Daumy I, Leux C, Bodet-Milin C, Kraeber-Bodéré F, Ansquer C (2008). "99mTc-MIBI pinhole SPECT in primary hyperparathyroidism: comparison with conventional SPECT, planar scintigraphy and ultrasonography". Eur. J. Nucl. Med. Mol. Imaging. 35 (3): 637–43. doi:10.1007/s00259-007-0625-9. PMC 2964350. PMID 17960377.
- ↑ Nguyen BD (1999). "Parathyroid imaging with Tc-99m sestamibi planar and SPECT scintigraphy". Radiographics. 19 (3): 601–14, discussion 615-6. doi:10.1148/radiographics.19.3.g99ma10601. PMID 10336191.
- ↑ Lindqvist V, Jacobsson H, Chandanos E, Bäckdahl M, Kjellman M, Wallin G (2009). "Preoperative 99Tc(m)-sestamibi scintigraphy with SPECT localizes most pathologic parathyroid glands". Langenbecks Arch Surg. 394 (5): 811–5. doi:10.1007/s00423-009-0536-2. PMID 19578871.
- ↑ Wimmer G, Profanter C, Kovacs P, Sieb M, Gabriel M, Putzer D; et al. (2010). "CT-MIBI-SPECT image fusion predicts multiglandular disease in hyperparathyroidism". Langenbecks Arch Surg. 395 (1): 73–80. doi:10.1007/s00423-009-0545-1. PMID 19705144.
- ↑ Tang BN, Moreno-Reyes R, Blocklet D, Corvilain B, Cappello M, Delpierre I; et al. (2008). "Accurate pre-operative localization of pathological parathyroid glands using 11C-methionine PET/CT". Contrast Media Mol Imaging. 3 (4): 157–63. doi:10.1002/cmmi.243. PMID 18781582.
- ↑ Weber T, Maier-Funk C, Ohlhauser D, Hillenbrand A, Cammerer G, Barth TF; et al. (2013). "Accurate preoperative localization of parathyroid adenomas with C-11 methionine PET/CT". Ann Surg. 257 (6): 1124–8. doi:10.1097/SLA.0b013e318289b345. PMID 23478517.
- ↑ Traub-Weidinger T, Mayerhoefer ME, Koperek O, Mitterhauser M, Duan H, Karanikas G; et al. (2014). "11C-methionine PET/CT imaging of 99mTc-MIBI-SPECT/CT-negative patients with primary hyperparathyroidism and previous neck surgery". J Clin Endocrinol Metab. 99 (11): 4199–205. doi:10.1210/jc.2014-1267. PMID 25029418.
- ↑ Wood K, Dhital S, Chen H, Sippel RS (2012). "What is the utility of distal forearm DXA in primary hyperparathyroidism?". Oncologist. 17 (3): 322–5. doi:10.1634/theoncologist.2011-0285. PMC 3316917. PMID 22258698.
- ↑ Nussbaum SR, Thompson AR, Hutcheson KA, Gaz RD, Wang CA (1988). "Intraoperative measurement of parathyroid hormone in the surgical management of hyperparathyroidism". Surgery. 104 (6): 1121–7. PMID 3194839.
- ↑ Bergenfelz A, Isaksson A, Ahrén B (1994). "Intraoperative monitoring of intact PTH during surgery for primary hyperparathyroidism". Langenbecks Arch Chir. 379 (1): 50–3. PMID 8145618.
- ↑ Irvin III, George L.; Dembrow, Victor D.; Prudhomme, David L. (December 1993). "Clinical usefulness of an intraoperative "quick parathyroid hormone" assay". Surgery. 114 (6): 1019–1023.
- ↑ Bergenfelz A, Isaksson A, Lindblom P, Westerdahl J, Tibblin S (1998). "Measurement of parathyroid hormone in patients with primary hyperparathyroidism undergoing first and reoperative surgery". Br J Surg. 85 (8): 1129–32. doi:10.1046/j.1365-2168.1998.00824.x. PMID 9718013.
- ↑ Boggs JE, Irvin GL, Molinari AS, Deriso GT (1996). "Intraoperative parathyroid hormone monitoring as an adjunct to parathyroidectomy" (PDF). Surgery. 120 (6): 954–8. doi:10.1016/S0039-6060(96)80040-7. PMID 8957480.
- ↑ Westerdahl J, Lindblom P, Bergenfelz A (2002). "Measurement of intraoperative parathyroid hormone predicts long-term operative success". Arch Surg. 137 (2): 186–90. doi:10.1001/archsurg.137.2.186. PMID 11822958.
- ↑ Powell AC, Alexander HR, Chang R, Marx SJ, Skarulis M, Pingpank JF; et al. (2009). "Reoperation for parathyroid adenoma: a contemporary experience". Surgery. 146 (6): 1144–55. doi:10.1016/j.surg.2009.09.015. PMC 3467310. PMID 19958942.
- ↑ Miller DL, Chang R, Doppman JL, Norton JA (1989). "Localization of parathyroid adenomas: superselective arterial DSA versus superselective conventional angiography". Radiology. 170 (3 Pt 2): 1003–6. doi:10.1148/radiology.170.3.2644666. PMID 2644666.