Sandbox : anmol
|
Hyperparathyroidism Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sandbox : anmol On the Web |
|
American Roentgen Ray Society Images of Sandbox : anmol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Anmol Pitliya, M.B.B.S. M.D.[2]
Classification
| Classification of hyperparathyridism | |||
|---|---|---|---|
| Features | Primary hyperparathyroidism | Secondary hyperparathyroidism | Tertiary hyperparathyroidism |
| Pathology | Hyperfunction of parathyroid cells due to hyperplasia, adenoma or carcinoma. | Physiological stimulation of parathyroid in response to hypocalcaemia. | Following long term physiological stimulation leading to hyperplasia. |
| Cause | |||
| Associations | May be associated with multiple endocrine neoplasia. | Usually due to chronic renal failure or other causes of Vitamin D deficiency. | Seen in chronic renal failure. |
| Serum calcium | High | Low/Normal | High |
| Serum phosphate | Low/Normal | High | High |
| Management | Usually surgery if symptomatic. Cincacalcet can be considered in those not fit for surgery. | Treatment of underlying cause. | Usually cinacalcet or surgery in those that don't respond. |
Causes
Overview
Hyperparathyroidism is caused by an increase in concentration of parathyroid hormone in serum. There are three type of hyperparathyroidism including primary, secondary and tertiary hyperparathyroidism. The are an array of different causes for all types of hyperparathyroidism.
Causes of Primary hyperparathyroidism
Causes of primary hyperparathyroidism are as follows:
Common causes
- Parathyroid adenoma
- Usually single gland affected
- Sometimes multiple gland affected
Less common causes
- Parathyroid hyperplasia
- Parathyroid carcinoma
- Familial isloated hyperparathyroidism
- Radiation exposure (due to development of parathyroid adenoma or parathyroid hyperplasia)[1][2][3]
- Celiac disease[4][5]
Genetic causes
- HRPT2 gene mutations:[6]
- HRPT2 gene code for parafibromin protein.
- HRPT2 gene mutations are found in a type of familial hyperparathyroidism, hyperparathyroidism-jaw tumor (HPT-JT) syndrome.
- HRTP2 gene mutations increases risk of parathyroid carcinoma.
- Cyclin D1 gene (CCND1)/PRAD1 gene:[7][8]
- PRAD1 (parathyroid adenoma 1) is a protooncogene located on chromosome 11q13.
- Cyclin D1 gene translocation and oncogene action observerd in 8% of adenomas
- Cyclin D1 gene overexpression is pbserved in 20% to 40% of parathyroid adenomas
- MEN1 gene:[7][9]
- MEN1 is a tumor supressor gene on chronosome 11q13.
- Somatic loss of single MEN1 allele is observed in 25% to 40% of sporadic parathyroid adenomas.
Causes of secondary hyperparathyroidism
Causes of secondary hyperparathyroidism are as follows:
Common causes
Less common causes
- Severe calcium deficiency[12]
- Gastric bypass surgery, particularly roux-en-Y gastric bypass (RYGBP)[13]
- Malabsorption syndrome[14]
Causes of tertiary hyperparathyroidism
Causes of tertiary hyperparathyroidism are as follows:
Common causes
- Chronic renal failure (leading to parathyroid hyperplasia)
- Renal transplant patients[15]
Less common cause
- Long standing celiac disease[4]
Pathogenesis
Associated conditions
- Hypercalcemia
- Chronic renal failure
- Osteitis fibrous cystica
- Osteoporosis
- Osteomalacia
- Osteoarthritis
- Brown tumor
- Multiple endocrine neoplasia type 1, type 2A, and type 4
- Familial isolated hyperparathyroidism
- Neonatal severe hyperparathyroidism
- Familial hypocalciuric hypercalcemia
- Hyperparathyroid-jaw tumor syndrome
- Pancreatitis[16]
Natural history, Prognosis and Complications
Natural history
- Primary hyperparathyroidism usually develops in the fifth decade of life, in post-menopausal women and starts as asymptomatic hypercalcemia in presence of increased parathyroid hormone.
- If left untreated, some of patients with primary hyperparathyroidism may commonly develop marked hypercalcemia, marked hypercalciuria, cortical bone demineralization and nephrolithiasis.[17][18]
- Secondary hyperparathyroidism arise in the early course of chronic renal failure. As renal failure progress, secondary hyperparathyroidism becomes more notable.[19]
- Secondary hyperparathyroidism leads to vascular calcification due to elevated calcium and phosphorus levels. This is strongly associated with increase in morbidity and mortality.[20]
- If left untreated, secondary hyperparathyroidism carries an increased risk of vascular calcification with increasing age and duration of dialysis in patients.
- Tertiary hyperparathyroidism usually develops in post renal transplant patients.[21]
- If left untreated, tertiary hyperparathyroidism in post renal transplant patients may carry the risk of amyloid deposition, calciphylaxis, destructive or erosive spondyloarthropathy, osteonecrosis, and musculoskeletal infections.
Complications
Primary hyperparathyroidism
Majority of complications of primary hyperparathyroidism are due to hypercalcemia. Common complications of primary hyperparathyroidism include:
- Bone related complication:[22][23]
- Brown tumor
- Osteitis fibrous cystica
- Osteoporosis
- Cardiac complications:[24]
- Aortic and mitral valve calcification
- Calcific deposits in the myocardium
- Left ventricular hypertrophy
- Endocrine complications:[16]
- Pancreatitis
- Gastrointestinal complications:[25]
- Peptic ulcer disease
- Metabolic complications:[26][27][25][11]
- Hypercalcemic crisis
- Osteomalacia
- Neuromuscular complications:
- Neuropathic muscle disease
- Pregnancy related complications:[28]
- Neonatal hypoparathyroidism
- Psychiatric complications:[29][30][31]
- Anxiety
- Cognitive dysfunction including verbal memory and nonverbal abstraction
- Depression
- Irritability
- Lack of concentration
- Sleep disturbances
- Renal complications:[17][32][33]
- Hypercalciuria
- Nephrolithiasis
- Nephrocalcinosis
- Renal insufficiency (impairement of GFR)
- Rheumatologic complications:[34][35][36]
- Gout
- Osteoarthritis
- Pseudogout
Secondary hyperparathyroidism
Complications of secondary hyperparathyroidism includes:
- Cardiovascular complications:[37]
- Impaired left ventricular diastolic function
- Left ventricular hypertrophy
- Hematologic complication:[38]
- Platlet function inhibition
- Metabolic complicattions:[39][40]
- Metabolic syndrome
- Musculoskeletal complications:[41][42][43]
- Renal Osteodystrophy
- Brown cysts
- Osteitis fibrosa cystica
- Osteoporosis
- Osteosclerosis
- Renal Osteodystrophy
- Neurologic complications:[44][45]
- Electroencephalogram abnormalities
- Uremic neuropathy
- Neuromuscular complications:[46]
- Neuropathic muscle disease
- System non-specific complications:[47]
- Metastatic calcifications
Tertiary hyperparathyroidism
Complications of tertiary hyperparathyroidism post renal transplantation includes:[21]
- Metabolic complications:[48]
- Calciphylaxis
- Musculoskeletal complications:
- Musculoskeletal infections
- Osteonecrosis
- Neuromuscular complications:[49]
- Neuropathic muscle disease
- Renal complications:[50]
- Nephrolithiasis
- Rheumatologic complications:[51]
- Destructive or erosive spondyloarthropathy
- System non-specific complications:
- Amyloid deposition
- Metastatic calcifications
Prognosis
- Prognosis of primary hyperparathyroidism is generally excellent after parathyroidectomy.
- The complications of primary hyperparathyroidism resolves after the treatment.
- Untreated complication of primary hyperparathyroidism may be fatal.[25]
- Effective treatment can reduce morbidity and mortality associated with uncontrolled secondary hyperparathyroidism.[20]
- Hyperphosphatemia and metastatic calcification results due elevated product of serum calcium and serum phosphorus. Both conditions are present in patients with secondary hyperparathyroidism in presence of end stage renal disease. This leads to a significant increase in morbidity and mortality. Aggressive control of hyperphosphatemia may improve prognosis[47].
- Prognosis of tertiary hyperparathyroidism is generally good after resection of abnormal hyperplastic gland.[52]
ECG
There are no CT scan findings associated with hyperparathyroidism. However, a CT scan may be helpful in the diagnosis of cardiac complications of hyperparathyroidism. Findings on ECG are due to hypercalcemia and includes:[53]
- ST segment - ST segment is short in patients with hyperparathyroidism when compared to normocalcemic patients. This represents a decrease in systolic interval.
- QRS complex - QRS complex has an increased amplitudein patients with hyperparathyroidism when compared to normocalcemic patients. This represents an increase in ventricular muscle mass.
- T wave - T wave is prolonged in patients with hyperparathyroidism when compared to normocalcemic patients.
X-ray
|
Finding in primary hyperparathyroidism includes:[54]
X-ray is the preferred imaging for diagnosis of secondary hyperparathyroidism as majority of findings are radiological. [55] Findings in secondary and tertiary hyperparathyroidism are often associated with the osteosclerosis of renal osteodystrophy, and the osteomalacia of vitamin D deficiency:
| |
 |
 |
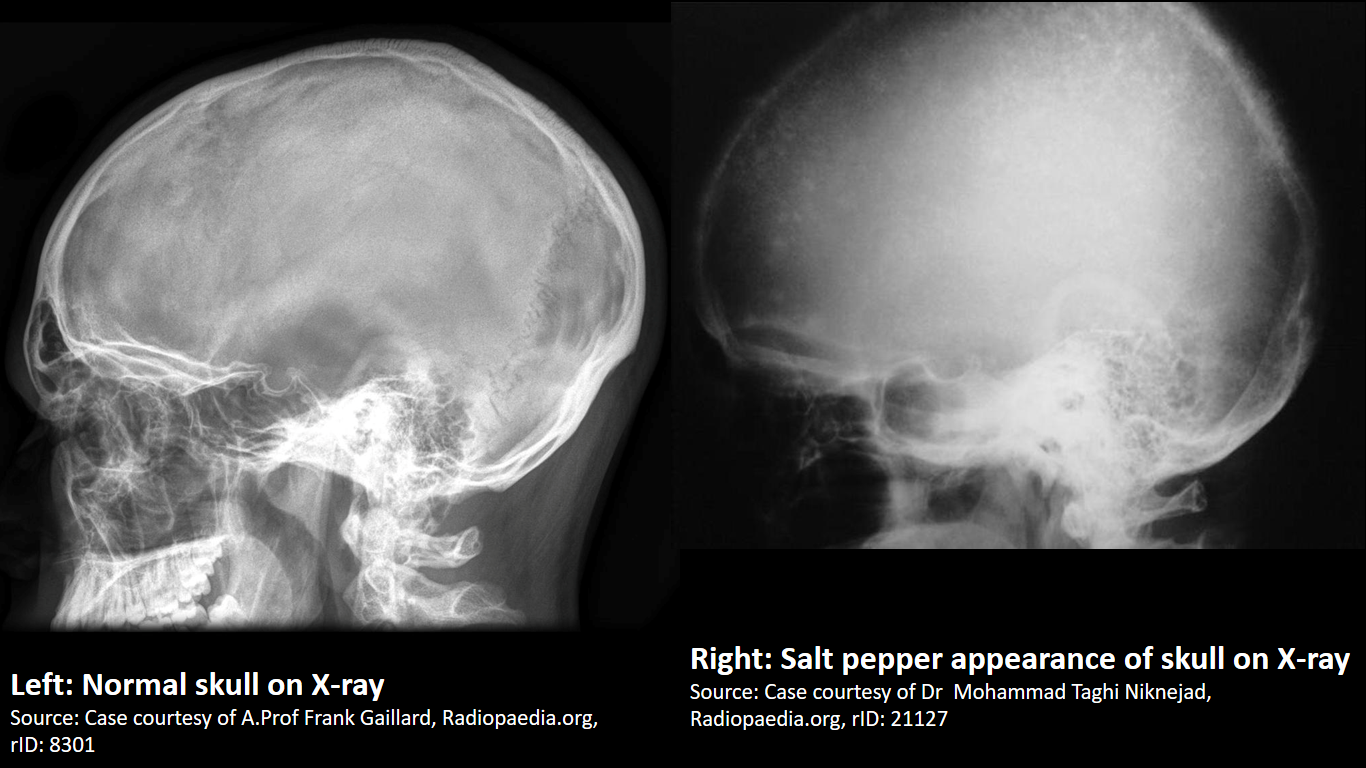 |
 |
Medical Therapy
Monitoring
Patients with primary hyperparathyroidism who do not undergo parathyroidectomy should be monitored for the potential progression of disease. There are guidelines for monitoring of patients with asymptomatic hyperparathyroidism not undergoing parathyroidectomy. These guidelines include:[56]
- Serum calcium
- Serum calcium should be monitored annually.
- Skeletal monitoring
- Dual-energy X-ray absorptiometry (DEXA) is used for skeletal monitoring. DEXA should be done every 1-2 years (at 3 sites).
- X-ray or vertebral fracture assessment of spine may be done if indications are present such as height loss, and/or back pain.
- Renal monitoring
- Estimated glomerular filtration rate (eGFR) and serum creatinine should be done annually.
- 24-h biochemical stone profile, renal imaging by x-ray, ultrasound, or CT scan may be considered if renal stones are suspected.
Medical Management
- 1. Primary hyperparathyroidism
- 1.1 Nutritional supplementation[57]
- 1.1.1 Low calcium intake[58]
- Preferred regimen (1): Elemental calcium 500 mg PO q24h
- Note: Dietary calcium restriction is not necessary in primary hyperparathyroidism.
- 1.1.2 Vitamin D depletion
- Preferred regimen (1): Cholecalciferol 600–1000 IU PO q24h
- Note(1): Vitamin D deficiency is considered when serum level of 25-hydroxy vitamin D is below 50 nM (20 ng/mL).[59]
- Note(2): Serum calcium levels and urinary calcium excretion should be monitored during vitamin D supplementation. Vitamin D supplementation should be stopped if serum calcium levels is >11.6 mg/dL and/or urinary calcium excretion is >400 mg/24 h.
- Note(3): The goal of vitamin D supplementation is to keep 25-hydroxy vitamin D level between 50 nmol/L to 75 nmol/L.
- 1.1.1 Low calcium intake[58]
- 1.2 Pharmacotherapy
- 1.2.1 Estrogen Receptor-Targeted Therapy (Post-menopausal women)
- Preferred regimen (1): Conjugated equine estrogen 0.625 mg q24h + medroxyprogesterone acetate 5mg q24h
- Note(1): The risk-benefit ratio should be assessed with respect to known relative or absolute contraindication to use of estrogen in each patient.
- 1.2.2 Bisphosphonates
- 1.2.3 Calcimimetics
- Note(1): Cincalcet may be used in patients with familial primary hyperparathyroidism as a treatment option for patients having recurrent or persistent hypercalcemia after parathyroidectomy.
- Note(2): A combination of bisphosphonates and calcimimetics may be used to reduce the serum calcium and improve BMD.[64]
- 1.2.1 Estrogen Receptor-Targeted Therapy (Post-menopausal women)
- 1.1 Nutritional supplementation[57]
- 2. Secondary hyperparathyroidism[65]
- 2.1 Secondary hyperparathyroidism due to vitamin D deficiency
- Preferred regimen (1): Vitamin D analogs
- 2.2 Secondary hyperparathyroidism due to Chronic renal failure
- 2.2.1 Calcimimetics[66]
- Preferred regimen (1): Cinacalcet HCL
- 2.2.2 Phosphate binders/Phosphate rstriction
- 2.2.3 Vitamin D analogs
- 2.2.1 Calcimimetics[66]
- 2.1 Secondary hyperparathyroidism due to vitamin D deficiency
References
- ↑ Boehm BO, Rosinger S, Belyi D, Dietrich JW (2011). "The parathyroid as a target for radiation damage". N Engl J Med. 365 (7): 676–8. doi:10.1056/NEJMc1104982. PMID 21848480.
- ↑ McMullen T, Bodie G, Gill A, Ihre-Lundgren C, Shun A, Bergin M; et al. (2009). "Hyperparathyroidism after irradiation for childhood malignancy". Int J Radiat Oncol Biol Phys. 73 (4): 1164–8. doi:10.1016/j.ijrobp.2008.06.1487. PMID 18774659.
- ↑ Tisell LE, Hansson G, Lindberg S, Ragnhult I (1977). "Hyperparathyroidism in persons treated with X-rays for tuberculous cervical adenitis". Cancer. 40 (2): 846–54. PMID 890665.
- ↑ 4.0 4.1 Maida MJ, Praveen E, Crimmins SR, Swift GL (2006). "Coeliac disease and primary hyperparathyroidism: an association?". Postgrad Med J. 82 (974): 833–5. doi:10.1136/pgmj.2006.045500. PMC 2653933. PMID 17148709.
- ↑ Ludvigsson JF, Kämpe O, Lebwohl B, Green PH, Silverberg SJ, Ekbom A (2012). "Primary hyperparathyroidism and celiac disease: a population-based cohort study". J. Clin. Endocrinol. Metab. 97 (3): 897–904. doi:10.1210/jc.2011-2639. PMC 3319223. PMID 22238405.
- ↑ Shattuck TM, Välimäki S, Obara T, Gaz RD, Clark OH, Shoback D; et al. (2003). "Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma". N Engl J Med. 349 (18): 1722–9. doi:10.1056/NEJMoa031237. PMID 14585940.
- ↑ 7.0 7.1 Westin G, Björklund P, Akerström G (2009). "Molecular genetics of parathyroid disease". World J Surg. 33 (11): 2224–33. doi:10.1007/s00268-009-0022-6. PMID 19373510.
- ↑ Hsi ED, Zukerberg LR, Yang WI, Arnold A (1996). "Cyclin D1/PRAD1 expression in parathyroid adenomas: an immunohistochemical study". J Clin Endocrinol Metab. 81 (5): 1736–9. doi:10.1210/jcem.81.5.8626826. PMID 8626826.
- ↑ Agarwal SK, Kester MB, Debelenko LV, Heppner C, Emmert-Buck MR, Skarulis MC; et al. (1997). "Germline mutations of the MEN1 gene in familial multiple endocrine neoplasia type 1 and related states". Hum Mol Genet. 6 (7): 1169–75. PMID 9215689.
- ↑ Rodriguez M, Nemeth E, Martin D (2005). "The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism". Am J Physiol Renal Physiol. 288 (2): F253–64. doi:10.1152/ajprenal.00302.2004. PMID 15507543.
- ↑ 11.0 11.1 Lips P (2001). "Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications". Endocr Rev. 22 (4): 477–501. doi:10.1210/edrv.22.4.0437. PMID 11493580.
- ↑ Mehrotra M, Gupta SK, Kumar K, Awasthi PK, Dubey M, Pandey CM; et al. (2006). "Calcium deficiency-induced secondary hyperparathyroidism and osteopenia are rapidly reversible with calcium supplementation in growing rabbit pups". Br J Nutr. 95 (3): 582–90. PMID 16512945.
- ↑ Johnson JM, Maher JW, DeMaria EJ, Downs RW, Wolfe LG, Kellum JM (2006). "The long-term effects of gastric bypass on vitamin D metabolism". Ann. Surg. 243 (5): 701–4, discussion 704–5. doi:10.1097/01.sla.0000216773.47825.c1. PMC 1570540. PMID 16633006.
- ↑ Pitt SC, Sippel RS, Chen H (2009). "Secondary and tertiary hyperparathyroidism, state of the art surgical management". Surg. Clin. North Am. 89 (5): 1227–39. doi:10.1016/j.suc.2009.06.011. PMC 2905047. PMID 19836494.
- ↑ Kilgo MS, Pirsch JD, Warner TF, Starling JR (1998). "Tertiary hyperparathyroidism after renal transplantation: surgical strategy". Surgery. 124 (4): 677–83, discussion 683–4. doi:10.1067/msy.1998.91483. PMID 9780988.
- ↑ 16.0 16.1 Bai HX, Giefer M, Patel M, Orabi AI, Husain SZ (2012). "The association of primary hyperparathyroidism with pancreatitis". J. Clin. Gastroenterol. 46 (8): 656–61. doi:10.1097/MCG.0b013e31825c446c. PMC 4428665. PMID 22874807.
- ↑ 17.0 17.1 Peacock M (2002). "Primary hyperparathyroidism and the kidney: biochemical and clinical spectrum". J. Bone Miner. Res. 17 Suppl 2: N87–94. PMID 12412783.
- ↑ Silverberg SJ, Shane E, de la Cruz L, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV (1989). "Skeletal disease in primary hyperparathyroidism". J. Bone Miner. Res. 4 (3): 283–91. doi:10.1002/jbmr.5650040302. PMID 2763869.
- ↑ Nikodimopoulou M, Liakos S (2011). "Secondary hyperparathyroidism and target organs in chronic kidney disease". Hippokratia. 15 (Suppl 1): 33–8. PMC 3139677. PMID 21897756.
- ↑ 20.0 20.1 Cunningham J, Locatelli F, Rodriguez M (2011). "Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options". Clin J Am Soc Nephrol. 6 (4): 913–21. doi:10.2215/CJN.06040710. PMID 21454719.
- ↑ 21.0 21.1 Jevtic V (2003). "Imaging of renal osteodystrophy". Eur J Radiol. 46 (2): 85–95. doi:10.1016/S0720-048X(03)00072-X. PMID 12714225.
- ↑ Bandeira F, Cusano NE, Silva BC, Cassibba S, Almeida CB, Machado VC, Bilezikian JP (2014). "Bone disease in primary hyperparathyroidism". Arq Bras Endocrinol Metabol. 58 (5): 553–61. PMC 4315357. PMID 25166047.
- ↑ Mazzuoli GF, D'Erasmo E, Pisani D (1998). "Primary hyperparathyroidism and osteoporosis". Aging (Milano). 10 (3): 225–31. PMID 9801732.
- ↑ Stefenelli T, Abela C, Frank H, Koller-Strametz J, Globits S, Bergler-Klein J, Niederle B (1997). "Cardiac abnormalities in patients with primary hyperparathyroidism: implications for follow-up". J. Clin. Endocrinol. Metab. 82 (1): 106–12. doi:10.1210/jcem.82.1.3666. PMID 8989242.
- ↑ 25.0 25.1 25.2 Corlew DS, Bryda SL, Bradley EL, DiGirolamo M (1985). "Observations on the course of untreated primary hyperparathyroidism". Surgery. 98 (6): 1064–71. PMID 3878002.
- ↑ Fitzpatrick LA, Bilezikian JP (1987). "Acute primary hyperparathyroidism". Am. J. Med. 82 (2): 275–82. PMID 3812520.
- ↑ Ahmad S, Kuraganti G, Steenkamp D (2015). "Hypercalcemic crisis: a clinical review". Am. J. Med. 128 (3): 239–45. doi:10.1016/j.amjmed.2014.09.030. PMID 25447624.
- ↑ Poomthavorn P, Ongphiphadhanakul B, Mahachoklertwattana P (2008). "Transient neonatal hypoparathyroidism in two siblings unmasking maternal normocalcemic hyperparathyroidism". Eur. J. Pediatr. 167 (4): 431–4. doi:10.1007/s00431-007-0528-6. PMID 17569990.
- ↑ Walker MD, McMahon DJ, Inabnet WB, Lazar RM, Brown I, Vardy S, Cosman F, Silverberg SJ (2009). "Neuropsychological features in primary hyperparathyroidism: a prospective study". J. Clin. Endocrinol. Metab. 94 (6): 1951–8. doi:10.1210/jc.2008-2574. PMC 2690425. PMID 19336505.
- ↑ Espiritu RP, Kearns AE, Vickers KS, Grant C, Ryu E, Wermers RA (2011). "Depression in primary hyperparathyroidism: prevalence and benefit of surgery". J. Clin. Endocrinol. Metab. 96 (11): E1737–45. doi:10.1210/jc.2011-1486. PMID 21917870.
- ↑ McAllion SJ, Paterson CR (1989). "Psychiatric morbidity in primary hyperparathyroidism". Postgrad Med J. 65 (767): 628–31. PMC 2429194. PMID 2608590.
- ↑ Lila AR, Sarathi V, Jagtap V, Bandgar T, Menon PS, Shah NS (2012). "Renal manifestations of primary hyperparathyroidism". Indian J Endocrinol Metab. 16 (2): 258–62. doi:10.4103/2230-8210.93745. PMC 3313745. PMID 22470864.
- ↑ Tassone F, Gianotti L, Emmolo I, Ghio M, Borretta G (2009). "Glomerular filtration rate and parathyroid hormone secretion in primary hyperparathyroidism". J. Clin. Endocrinol. Metab. 94 (11): 4458–61. doi:10.1210/jc.2009-0587. PMID 19808852.
- ↑ Michael JW, Schlüter-Brust KU, Eysel P (2010). "The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee". Dtsch Arztebl Int. 107 (9): 152–62. doi:10.3238/arztebl.2010.0152. PMC 2841860. PMID 20305774.
- ↑ Hochberg, Marc (2015). "204. Primary hyperparathyroidism: rheumatologic manifestations and bone disease". Rheumatology. Philadelphia, PA: Mosby/Elsevier. p. 1668. ISBN 9780323091381.
- ↑ Rubin MR, Silverberg SJ (2002). "Rheumatic manifestations of primary hyperparathyroidism and parathyroid hormone therapy". Curr Rheumatol Rep. 4 (2): 179–85. PMID 11890884.
- ↑ Strózecki P, Adamowicz A, Nartowicz E, Odrowaz-Sypniewska G, Włodarczyk Z, Manitius J (2001). "Parathormon, calcium, phosphorus, and left ventricular structure and function in normotensive hemodialysis patients". Ren Fail. 23 (1): 115–26. PMID 11256521.
- ↑ Remuzzi G, Benigni A, Dodesini P, Schieppati A, Livio M, Poletti E, Mecca G, de Gaetano G (1981). "Parathyroid hormone inhibits human platelet function". Lancet. 2 (8259): 1321–3. doi:10.1016/S0140-6736(81)91343-X. PMID 6118720.
- ↑ Saab G, Whaley-Connell A, Bombeck A, Kurella Tamura M, Li S, Chen SC, McFarlane SI, Sowers JR, Norris K, Bakris GL, McCullough PA (2011). "The Association between Parathyroid Hormone Levels and the Cardiorenal Metabolic Syndrome in Non-Diabetic Chronic Kidney Disease". Cardiorenal Med. 1 (2): 123–130. doi:10.1159/000327149. PMC 3101512. PMID 22258399.
- ↑ Hjelmesæth, Jøran; Hofsø, Dag; Aasheim, Erlend T; Jenssen, Trond; Moan, Johan; Hager, Helle; Røislien, Jo; Bollerslev, Jens (2009). "Parathyroid hormone, but not vitamin D, is associated with the metabolic syndrome in morbidly obese women and men: a cross-sectional study". Cardiovascular Diabetology. 8 (1): 7. doi:10.1186/1475-2840-8-7. ISSN 1475-2840.
- ↑ Spaulding CM, Young G (1997). "Osteitis fibrosa cystica and chronic renal failure". J Am Podiatr Med Assoc. 87 (5): 238–40. doi:10.7547/87507315-87-5-238. PMID 9158318.
- ↑ Eastwood JB (1977). "Renal osteodystrophy--a radiological review". CRC Crit Rev Diagn Imaging. 9 (1): 77–104. PMID 328228.
- ↑ Adams JE (1999). "Renal bone disease: radiological investigation". Kidney Int. Suppl. 73: S38–41. PMID 10633462.
- ↑ Goldstein DA, Feinstein EI, Chui LA, Pattabhiraman R, Massry SG (1980). "The relationship between the abnormalities in electroencephalogram and blood levels of parathyroid hormone in dialysis patients". J. Clin. Endocrinol. Metab. 51 (1): 130–4. doi:10.1210/jcem-51-1-130. PMID 6892917.
- ↑ Avram MM, Feinfeld DA, Huatuco AH (1978). "Search for the uremic toxin. Decreased motor-nerve conduction velocity and elevated parathyroid hormone in uremia". N. Engl. J. Med. 298 (18): 1000–3. doi:10.1056/NEJM197805042981805. PMID 205786.
- ↑ Mallette LE, Patten BM, Engel WK (1975). "Neuromuscular disease in secondary hyperparathyroidism". Ann. Intern. Med. 82 (4): 474–83. PMID 47234.
- ↑ 47.0 47.1 Block GA, Hulbert-Shearon TE, Levin NW, Port FK (1998). "Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study". Am. J. Kidney Dis. 31 (4): 607–17. PMID 9531176.
- ↑ Barbur MA, Kurjak M, Becker K (1997). "[Systematic calciphylaxis in chronic renal failure: fulminant course after kidney transplantation]". Pathologe (in German). 18 (6): 453–8. PMID 9451734.
- ↑ Gerhardt RE, Zeitlin EL (1978). "Neuromuscular disease in tertiary hyperparathyroidism". Arch. Intern. Med. 138 (6): 1013–5. PMID 646555.
- ↑ Kim H, Cheigh JS, Ham HW (2001). "Urinary stones following renal transplantation". Korean J. Intern. Med. 16 (2): 118–22. PMC 4531707. PMID 11590898.
- ↑ Adler JS, Cameron DC (1989). "Erosive spondylo-arthropathy and tertiary hyperparathyroidism". Australas Radiol. 33 (1): 90–2. PMID 2712794.
- ↑ Nichol PF, Starling JR, Mack E, Klovning JJ, Becker BN, Chen H (2002). "Long-term follow-up of patients with tertiary hyperparathyroidism treated by resection of a single or double adenoma". Ann. Surg. 235 (5): 673–8, discussion 678–80. PMC 1422493. PMID 11981213.
- ↑ Lind L, Ljunghall S (1994). "Serum calcium and the ECG in patients with primary hyperparathyroidism". J Electrocardiol. 27 (2): 99–103. PMID 8201301.
- ↑ Lachungpa T, Sarawagi R, Chakkalakkoombil SV, Jayamohan AE (2014). "Imaging features of primary hyperparathyroidism". BMJ Case Rep. 2014. doi:10.1136/bcr-2013-203521. PMC 3962932. PMID 24614783.
- ↑ Tigges S, Nance EP, Carpenter WA, Erb R (1995). "Renal osteodystrophy: imaging findings that mimic those of other diseases". AJR Am J Roentgenol. 165 (1): 143–8. doi:10.2214/ajr.165.1.7785573. PMID 7785573.
- ↑ Bilezikian JP, Brandi ML, Eastell R, Silverberg SJ, Udelsman R, Marcocci C; et al. (2014). "Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop". J Clin Endocrinol Metab. 99 (10): 3561–9. doi:10.1210/jc.2014-1413. PMC 5393490. PMID 25162665.
- ↑ Marcocci C, Bollerslev J, Khan AA, Shoback DM (2014). "Medical management of primary hyperparathyroidism: proceedings of the fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism". J Clin Endocrinol Metab. 99 (10): 3607–18. doi:10.1210/jc.2014-1417. PMID 25162668.
- ↑ Jorde R, Szumlas K, Haug E, Sundsfjord J (2002). "The effects of calcium supplementation to patients with primary hyperparathyroidism and a low calcium intake". Eur J Nutr. 41 (6): 258–63. doi:10.1007/s00394-002-0383-1. PMID 12474069.
- ↑ Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK; et al. (2011). "The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know". J Clin Endocrinol Metab. 96 (1): 53–8. doi:10.1210/jc.2010-2704. PMC 3046611. PMID 21118827.
- ↑ Chow CC, Chan WB, Li JK, Chan NN, Chan MH, Ko GT; et al. (2003). "Oral alendronate increases bone mineral density in postmenopausal women with primary hyperparathyroidism". J Clin Endocrinol Metab. 88 (2): 581–7. doi:10.1210/jc.2002-020890. PMID 12574184.
- ↑ Khan AA, Bilezikian JP, Kung AW, Ahmed MM, Dubois SJ, Ho AY; et al. (2004). "Alendronate in primary hyperparathyroidism: a double-blind, randomized, placebo-controlled trial". J Clin Endocrinol Metab. 89 (7): 3319–25. doi:10.1210/jc.2003-030908. PMID 15240609.
- ↑ Peacock M, Bilezikian JP, Klassen PS, Guo MD, Turner SA, Shoback D (2005). "Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism". J Clin Endocrinol Metab. 90 (1): 135–41. doi:10.1210/jc.2004-0842. PMID 15522938.
- ↑ Luque-Fernández I, García-Martín A, Luque-Pazos A (2013). "Experience with cinacalcet in primary hyperparathyroidism: results after 1 year of treatment". Ther Adv Endocrinol Metab. 4 (3): 77–81. doi:10.1177/2042018813482344. PMC 3666442. PMID 23730501.
- ↑ Faggiano A, Di Somma C, Ramundo V, Severino R, Vuolo L, Coppola A; et al. (2011). "Cinacalcet hydrochloride in combination with alendronate normalizes hypercalcemia and improves bone mineral density in patients with primary hyperparathyroidism". Endocrine. 39 (3): 283–7. doi:10.1007/s12020-011-9459-0. PMID 21445714.
- ↑ Wetmore JB, Quarles LD (2009). "Calcimimetics or vitamin D analogs for suppressing parathyroid hormone in end-stage renal disease: time for a paradigm shift?". Nat Clin Pract Nephrol. 5 (1): 24–33. doi:10.1038/ncpneph0977. PMC 3924719. PMID 18957950.
- ↑ Strippoli GF, Palmer S, Tong A, Elder G, Messa P, Craig JC (2006). "Meta-analysis of biochemical and patient-level effects of calcimimetic therapy". Am J Kidney Dis. 47 (5): 715–26. doi:10.1053/j.ajkd.2006.01.015. PMID 16632010.