Sandbox : anmol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Lyme disease}} | {{Lyme disease}} | ||
{{CMG}} {{AE}} {{IMD}} | {{CMG}};{{AE}} {{Anmol}},{{IMD}} | ||
==Overview== | ==Overview== | ||
Laboratory blood tests are helpful if used correctly and performed with validated methods. Laboratory tests are not recommended for patients who do not have symptoms typical of Lyme disease. [[Polymerase chain reaction]] (PCR) tests for Lyme disease have also been developed to detect the genetic material ([[DNA]]) of the Lyme disease spirochete. | Laboratory blood tests are helpful if used correctly and performed with validated methods. Laboratory tests are not recommended for patients who do not have symptoms typical of Lyme disease. [[Polymerase chain reaction]] (PCR) tests for Lyme disease have also been developed to detect the genetic material ([[DNA]]) of the Lyme disease spirochete. Currently, PCR is the only means to detect the presence of organism. Identification and testing of individual tick after removal is generally not recommended. | ||
==Laboratory Findings== | ==Laboratory Findings== | ||
| Line 14: | Line 14: | ||
===Serology=== | ===Serology=== | ||
==== Two-step Laboratory Testing Process<ref name="urlTwo-step Laboratory Testing Process| Lyme Disease | CDC">{{cite web |url=https://www.cdc.gov/lyme/diagnosistesting/labtest/twostep/index.html |title=Two-step Laboratory Testing Process| Lyme Disease | CDC |format= |work= |accessdate=}}</ref> ==== | ==== Two-step Laboratory Testing Process<ref name="urlTwo-step Laboratory Testing Process| Lyme Disease | CDC">{{cite web |url=https://www.cdc.gov/lyme/diagnosistesting/labtest/twostep/index.html |title=Two-step Laboratory Testing Process| Lyme Disease | CDC |format= |work= |accessdate=}}</ref> ==== | ||
*The [[serology|serological]] laboratory tests most widely available and employed are the [[Western blot]] and [[ELISA]]. | *The [[serology|serological]] laboratory tests most widely available and employed are the [[Western blot]] and [[ELISA]]. | ||
Revision as of 19:26, 1 August 2017
|
Lyme disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Sandbox : anmol On the Web |
|
American Roentgen Ray Society Images of Sandbox : anmol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Anmol Pitliya, M.B.B.S. M.D.[2],Ilan Dock, B.S.
Overview
Laboratory blood tests are helpful if used correctly and performed with validated methods. Laboratory tests are not recommended for patients who do not have symptoms typical of Lyme disease. Polymerase chain reaction (PCR) tests for Lyme disease have also been developed to detect the genetic material (DNA) of the Lyme disease spirochete. Currently, PCR is the only means to detect the presence of organism. Identification and testing of individual tick after removal is generally not recommended.
Laboratory Findings
- Lyme disease is diagnosed based on:
- Signs and symptoms
- A history of possible exposure to infected blacklegged ticks
- Laboratory blood tests are helpful if used correctly and performed with validated methods.
- Laboratory tests are not recommended for patients who do not have symptoms typical of Lyme disease.
- Just as it is important to correctly diagnose Lyme disease when a patient has it, it is important to avoid misdiagnosis and treatment of Lyme disease when the true cause of the illness is something else.
Serology
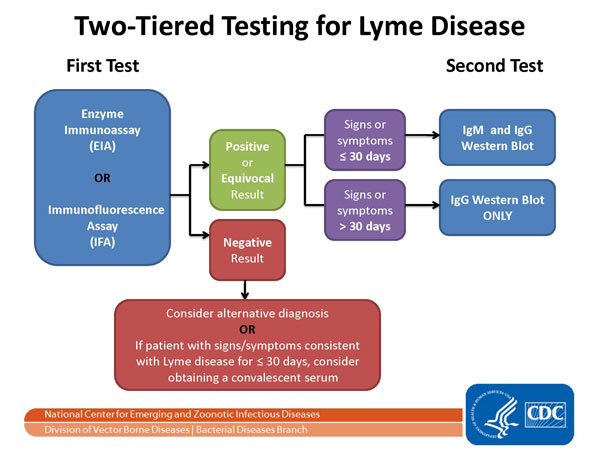
Two-step Laboratory Testing Process[1]
- The serological laboratory tests most widely available and employed are the Western blot and ELISA.
- A two-tiered protocol is recommended by the CDC: the more sensitive ELISA is performed first, if it is positive or equivocal, the more specific Western blot is run. The reliability of testing in diagnosis remains controversial, however studies show the Western blot IgM has a specificity of 94–96% for patients with clinical symptoms of early Lyme disease.[2][3]
- The two steps of Lyme disease testing are designed to be done together. CDC does not recommend skipping the first test and just doing the Western blot. Doing so will increase the frequency of false positive results and may lead to misdiagnosis and improper treatment.
- Erroneous test results have been widely reported in both early and late stages of the disease. These errors can be caused by several factors, including antibody cross-reactions from other infections including
- Epstein-Barr virus[4][5]
- Cytomegalovirus[4]
- Varicella zoster virus[6][7]
- Herpes simplex virus type 2[8]
- Rickettsial infections[5]
- Syphilis[5]
Polymerase chain reaction
- Polymerase chain reaction (PCR) tests for Lyme disease have also been developed to detect the genetic material (DNA) of the Lyme disease spirochete. PCR tests are rarely susceptible to false-positive results but can often show false-negative results, and the overall reliability of PCR in this role remains unclear.
- With the exception of PCR, there is no currently practical means for detection of the presence of the organism, as serologic studies only test for antibodies of Borrelia.
- High titers of either immunoglobulin G (IgG) or immunoglobulin M (IgM) antibodies to Borrelia antigens indicate disease, but lower titers can be misleading.
- The IgM antibodies may remain after the initial infection, and IgG antibodies may remain for years.[9]
- Western blot, ELISA and PCR can be performed by either blood test via venipuncture or cerebral spinal fluid (CSF) via lumbar puncture.
- Though lumbar puncture is more definitive of diagnosis, antigen capture in the CSF is much more elusive, reportedly CSF yields positive results in only 10-30% of patients cultured.
- The diagnosis of neurologic infection by Borrelia should not be excluded solely on the basis of normal routine CSF or negative CSF antibody analyses.[10]
- New techniques for clinical evaluation if Borrelia infection are under investigation, and includes:
- New research indicates chemokine CXCL13 may also be a possible marker for neuroborreliosis.[13]
Other Types of Laboratory Testing
- Some laboratories offer Lyme disease testing using assays whose accuracy and clinical usefulness have not been adequately established. These tests include:[14]
- Capture assays for antigens in urine
- Culture, immunofluorescence staining, or cell sorting of cell wall-deficient or cystic forms of B. burgdorferi
- Lymphocyte transformation tests
- Quantitative CD57 lymphocyte assays
- “Reverse Western blots”
- In-house criteria for interpretation of immunoblots
- Measurements of antibodies in joint fluid (synovial fluid)
- IgM or IgG tests without a previous ELISA/EIA/IFA
- In general, CDC does not recommend these tests.
- Patients are encouraged to ask their physicians whether their testing for Lyme disease was performed using validated methods and whether results were interpreted using appropriate guidelines.
Testing Ticks
- Patients who have removed a tick often wonder if they should have it tested.
- In general, the identification and testing of individual ticks is not useful for deciding if a person should get antibiotics following a tick bite because:[15]
- If the test shows that the tick contained disease-causing organisms, that does not necessarily mean that the patient have been infected.
- If the patient have been infected, symptoms will develop probably before results of the tick test are available. So, appropriate treatment should not be withhold for availability tick testing results.
- Negative results can lead to false assurance. For example, the patient may have been unknowingly bitten by a different tick that was infected.
References
- ↑ "Two-step Laboratory Testing Process| Lyme Disease | CDC".
- ↑ Engstrom SM, Shoop E, Johnson RC (1995). "Immunoblot interpretation criteria for serodiagnosis of early Lyme disease" (PDF). J Clin Microbiol. 33 (2): 419–27. PMID 7714202.
- ↑ Sivak SL, Aguero-Rosenfeld ME, Nowakowski J, Nadelman RB, Wormser GP (1996). "Accuracy of IgM immunoblotting to confirm the clinical diagnosis of early Lyme disease". Arch Intern Med. 156 (18): 2105–9. PMID 8862103.
- ↑ 4.0 4.1 Goossens HA, Nohlmans MK, van den Bogaard AE (1999). "Epstein-Barr virus and cytomegalovirus infections cause false-positive results in IgM two-test protocol for early Lyme borreliosis". Infection. 27 (3): 231. PMID 10378140.
- ↑ 5.0 5.1 5.2 Berardi VP, Weeks KE, Steere AC (1988). "Serodiagnosis of early Lyme disease: analysis of IgM and IgG antibody responses by using an antibody-capture enzyme immunoassay". J Infect Dis. 158 (4): 754–60. PMID 3049839.
- ↑ Feder HM, Gerber MA, Luger SW, Ryan RW (1991). "False positive serologic tests for Lyme disease after varicella infection". N Engl J Med. 325 (26): 1886–7. PMID 1961232.
- ↑ Woelfle J, Wilske B, Haverkamp F, Bialek R (1998). "False-positive serological tests for Lyme disease in facial palsy and varicella zoster meningo-encephalitis". Eur J Pediatr. 157 (11): 953–4. PMID 9835449.
- ↑ Strasfeld L, Romanzi L, Seder RH, Berardi VP (2005). "False-positive serological test results for Lyme disease in a patient with acute herpes simplex virus type 2 infection". Clin Infect Dis. 41 (12): 1826–7. PMID 16288417.
- ↑ Burdash N, Fernandes J (1991). "Lyme borreliosis: detecting the great imitator". The Journal of the American Osteopathic Association. 91 (6): 573–4, 577–8. PMID 1874654.
- ↑ Coyle PK, Schutzer SE, Deng Z; et al. (1995). "Detection of Borrelia burgdorferi-specific antigen in antibody-negative cerebrospinal fluid in neurologic Lyme disease". Neurology. 45 (11): 2010–5. PMID 7501150.
- ↑ Valentine-Thon E, Ilsemann K, Sandkamp M (2007). "A novel lymphocyte transformation test (LTT-MELISA) for Lyme borreliosis". Diagn. Microbiol. Infect. Dis. 57 (1): 27–34. doi:10.1016/j.diagmicrobio.2006.06.008. PMID 16876371.
- ↑ Eisendle K, Grabner T, Zelger B (2007). "Focus floating microscopy: "gold standard" for cutaneous borreliosis?". Am. J. Clin. Pathol. 127 (2): 213–22. doi:10.1309/3369XXFPEQUNEP5C. PMID 17210530.
- ↑ Cadavid D (2006). "The mammalian host response to borrelia infection". Wien. Klin. Wochenschr. 118 (21–22): 653–8. doi:10.1007/s00508-006-0692-0. PMID 17160603.
- ↑ "Laboratory tests that are not recommended| Lyme Disease | CDC".
- ↑ "Tick removal and testing | Lyme Disease | CDC".