Rivaroxaban
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ahmed Zaghw, M.D. [2]
For patient information, click here. Synonyms / Brand Names: XARELTO®
Overview
Rivaroxaban (BAY 59-7939) is an oral anticoagulant invented and manufactured by Bayer; in a number of countries it is marketed as Xarelto.[1] In the United States, it is marketed by Janssen Pharmaceutica.[2] It is an orally active direct factor Xa inhibitor. Rivaroxaban is well absorbed from the gut and maximum inhibition of factor Xa occurs four hours after a dose. The effects lasts 8 to 12 hours, but factor Xa activity does not return to normal within 24 hours so once-daily dosing is possible.
Category
Direct Xa factor inhibitor.
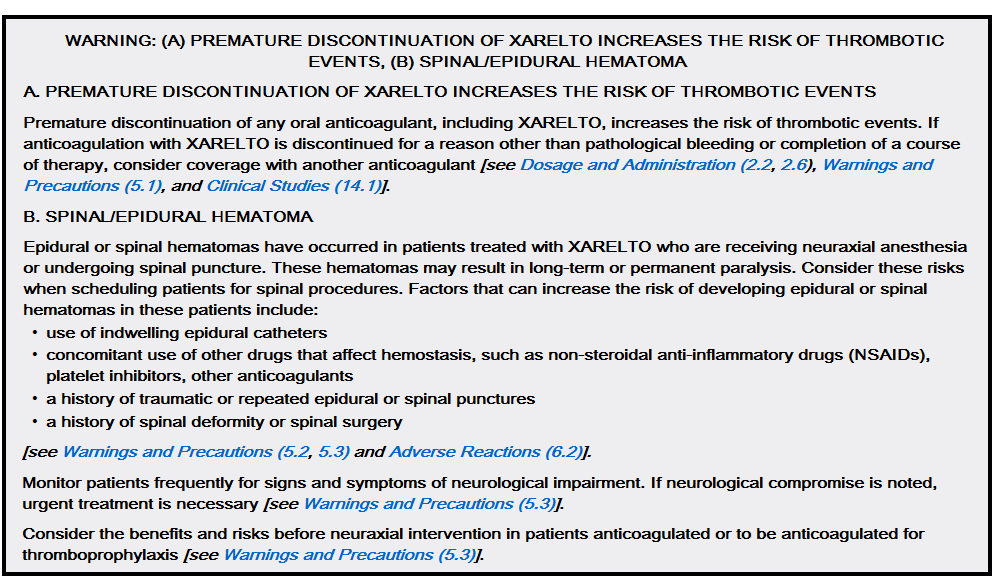
FDA Package Insert
XARELTO®
| Indications and Usage | Dosage and Administration | Dosage Forms and Strengths | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Use in Specific Populations | Overdosage | Description | Clinical Pharmacology | Nonclinical Toxicology | Clinical Studies | How Supplied/Storage and Handling | Patient Counseling Information | Labels and Packages
Mechanism of Action
XARELTO is a selective inhibitor of FXa. It does not require a cofactor (such as Anti-thrombin III) for activity. Rivaroxaban inhibits free FXa and prothrombinase activity. Rivaroxaban has no direct effect on platelet aggregation, but indirectly inhibits platelet aggregation induced by thrombin. By inhibiting FXa, rivaroxaban decreases thrombin generation.
References
- ↑ "Xarelto: Summary of Product Characteristics". Bayer Schering Pharma AG. 2008. Retrieved 2009-02-11.
- ↑ "FDA Approves XARELTO® (rivaroxaban tablets) to Help Prevent Deep Vein Thrombosis in Patients Undergoing Knee or Hip Replacement Surgery" (Press release). Janssen Pharmaceutica. 2011-07-01. Retrieved 2011-07-01.