Rhinoscleroma laboratory findings
|
Rhinoscleroma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Rhinoscleroma laboratory findings On the Web |
|
American Roentgen Ray Society Images of Rhinoscleroma laboratory findings |
|
Risk calculators and risk factors for Rhinoscleroma laboratory findings |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Laboratory Findings
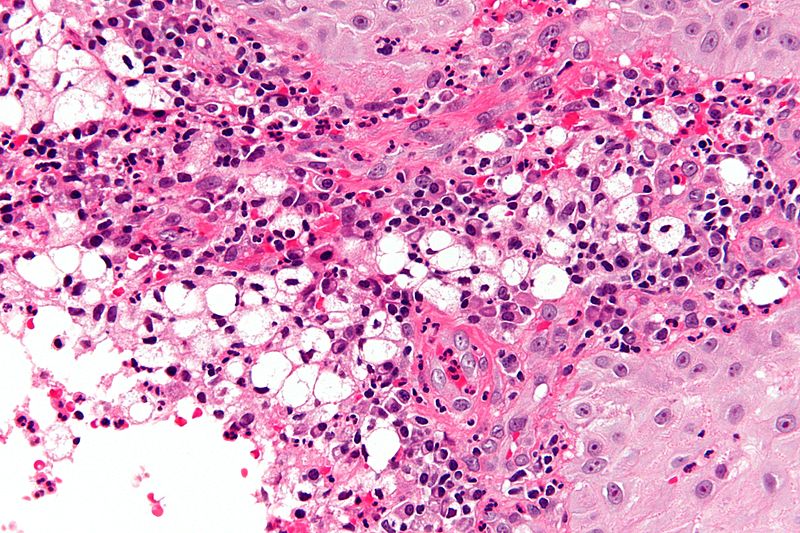
A positive culture in MacConkey agar is diagnostic, but cultures are only positive in 50-60% of cases. Diagnostic characteristics are most commonly found in the granulomatous stage and are described as being chronic inflammatory cells, Russell bodies, and pseudoepitheliomatous hyperplasia, and groups of large vacuolated histiocytes containing Klebsiella rhinoscleromatis (Mikulicz cells).