Regorafenib: Difference between revisions
(Created page with "__NOTOC__ {{Drugbox | drug_name = | IUPAC_name = 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-''N''-methylpyridine-2-carboxamide...") |
No edit summary |
||
| Line 3: | Line 3: | ||
| drug_name = | | drug_name = | ||
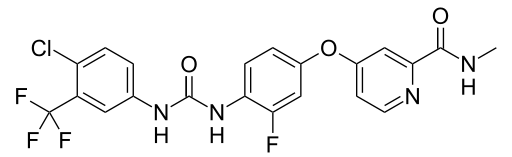
| IUPAC_name = 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-''N''-methylpyridine-2-carboxamide hydrate | | IUPAC_name = 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-''N''-methylpyridine-2-carboxamide hydrate | ||
| image = Regorafenib.svg | | image = Regorafenib.svg.png | ||
| alt = | | alt = | ||
| caption = | | caption = | ||
Revision as of 16:50, 30 October 2012
 | |
| Clinical data | |
|---|---|
| Trade names | Stivarga |
| Synonyms | BAY 73-4506 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H17ClF4N4O4 |
| Molar mass | 482.82 g mol |
| 3D model (JSmol) | |
| |
| |
|
WikiDoc Resources for Regorafenib |
|
Articles |
|---|
|
Most recent articles on Regorafenib Most cited articles on Regorafenib |
|
Media |
|
Powerpoint slides on Regorafenib |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Regorafenib at Clinical Trials.gov Clinical Trials on Regorafenib at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Regorafenib
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Regorafenib Discussion groups on Regorafenib Patient Handouts on Regorafenib Directions to Hospitals Treating Regorafenib Risk calculators and risk factors for Regorafenib
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Regorafenib |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Regorafenib (BAY 73-4506, commercial name Stivarga) is an oral multi-kinase inhibitor developed by Bayer which targets angiogenic, stromal and oncogenic receptor tyrosine kinase (RTK). Regorafenib shows anti-angiogenic activity due to its dual targeted VEGFR2-TIE2 tyrosine kinase inhibition. It is currently being studied as a potential treatment option in multiple tumor types.[1]
Regorafenib demonstrated to increase the overall survival of patients with metastatic colorectal cancer[2] and has been approved by the Food and Drug Administration on September 27, 2012.[3]
Stivarga is being approved with a Boxed Warning alerting patients and health care professionals that severe and fatal liver toxicity occurred in patients treated with Stivarga during clinical studies. The most common side effects reported in patients treated with Stivarga include weakness or fatigue, loss of appetite, hand-foot syndrome (also called palmar-plantar erythrodysesthesia), diarrhea, mouth sores (mucositis), weight loss, infection, high blood pressure, and changes in voice volume or quality (dysphonia).[4]
References
- ↑ "Bayer Announces New Data on Oncology Portfolio To Be Presented at the ECCO-ESMO Congress 2009". Retrieved 2009-09-19.
- ↑ "Phase III Trial of Regorafenib in Metastatic Colorectal Cancer Meets Primary Endpoint of Improving Overall Survival". Retrieved 2011-10-26.
- ↑ "FDA approves new treatment for advanced colorectal cancer". 27 Sep 2012.
- ↑ "FDA Prescribing Information" (PDF). 27 Sept 2012. Check date values in:
|date=(help)
- Pages with script errors
- CS1 errors: dates
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without UNII source
- Drugs missing an ATC code
- Articles containing unverified chemical infoboxes
- Receptor tyrosine kinase inhibitors
- Pyridines