Quinidine sulfate: Difference between revisions
No edit summary |
No edit summary |
||
| Line 245: | Line 245: | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
* '''Pregnancy Category C''' | * '''Pregnancy Category C''' | ||
:* | :* Animal reproductive studies have not been conducted with quinidine. There are no adequate and well-controlled studies in pregnant women. Quinidine should be given to a pregnant woman only if clearly needed. | ||
:* In one neonate whose mother had received quinidine throughout her pregnancy, the serum level of quinidine was equal to that of the mother, with no apparent ill effect. The level of quinidine in amniotic fluid was about three times higher than that found in serum. | |||
|useInPregnancyAUS= | |useInPregnancyAUS= | ||
| Line 254: | Line 255: | ||
|useInLaborDelivery= | |useInLaborDelivery= | ||
* Quinine is said to be oxytocic in humans, but there are no adequate data as to quinidine’s effects (if any) on human labor and delivery. | |||
|useInNursing= | |useInNursing= | ||
* Quinidine is present in human milk at levels slightly lower than those in maternal serum; a human infant ingesting such milk should (scaling directly by weight) be expected to develop serum quinidine levels at least an order of magnitude lower than those of the mother. On the other hand, the pharmacokinetics and pharmacodynamics of quinidine in human infants have not been adequately studied, and neonates’ reduced protein binding of quinidine may increase their risk of toxicity at low total serum levels. Administration of quinidine should (if possible) be avoided in lactating women who continue to nurse. | |||
|useInPed= | |useInPed= | ||
* In antimalarial trials, quinidine was as safe and effective in pediatric patients as in adults. Notwithstanding the known pharmacokinetic differences between the pediatric population and adults (see CLINICAL PHARMACOLOGY, Pharmacokinetics), pediatric patients in these trials received the same doses (on a mg/kg basis) as adults. | |||
* Safety and effectiveness of the antiarrhythmic use of quinidine in pediatric patients have not been established in well-controlled clinical trials. | |||
|useInGeri= | |useInGeri= | ||
* Clinical studies of quinidine generally were not adequate to determine if significant safety or efficacy differences exist between elderly patients (65 years or older) and younger patients. | |||
Quinidine clearance is apparently independent of age (see CLINICAL PHARMACOLOGY, Pharmacokinetics). However, renal or hepatic dysfunction causes the elimination of quinidine to be slowed (see WARNINGS, Pharmacokinetic Considerations), and since these conditions are more common in the elderly, appropriate dosing reductions should be considered in these individuals. | |||
|useInGender= | |useInGender= | ||
Revision as of 18:17, 13 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNINGS
See full prescribing information for complete Boxed Warning.
Mortality:
|
Overview
Quinidine sulfate is an antiarrhythmic that is FDA approved for the {{{indicationType}}} of symptomatic atrial fibrillation/flutter and life-threatening ventricular arrhythmias. There is a Black Box Warning for this drug as shown here. Common adverse reactions include chest pain, palpitations, rash, diarrhea, esophagitis, heartburn, nausea, vomiting, lightheadedness, visual disturbance, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Conversion of Atrial Fibrillation/Flutter to Sinus Rhythm
- Especially in patients with known structural heart disease or other risk factors for toxicity, initiation or dose-adjustment of treatment with quinidine sulfate should generally be performed in a setting where facilities and personnel for monitoring and resuscitation are continuously available. Patients with symptomatic atrial fibrillation/flutter should be treated with quinidine sulfate only after ventricular rate control (e.g., with digitalis or β-blockers) has failed to provide satisfactory control of symptoms. Adequate trials have not identified an optimal regimen of quinidine sulfate for conversion of atrial fibrillation/flutter to sinus rhythm.
- Dosing Information
- Therapy with quinidine sulfate should begin with one tablet (300 mg; 249 mg of quinidine base) every 8 to 12 hours.
- If this regimen is well tolerated, if the serum quinidine level is still well within the laboratory’s therapeutic range, and if this regimen has not resulted in conversion, then the dose may be cautiously raised. If, at any point during administration, the QRS complex widens to 130% of its pre-treatment duration; the QTc interval widens to 130% of its pre-treatment duration and is then longer than 500 ms; P waves disappear; or the patient develops significant tachycardia, symptomatic bradycardia, or hypotension, then quinidine sulfate is discontinued, and other means of conversion (e.g., direct-current cardioversion) are considered.
Reduction of Frequency of Relapse into Atrial Fibrillation/Flutter
- In a patient with a history of frequent symptomatic episodes of atrial fibrillation/flutter, the goal of therapy with quinidine sulfate should be an increase in the average time between episodes. In most patients, the tachyarrhythmia will recur during therapy with quinidine sulfate, and a single recurrence should not be interpreted as therapeutic failure.
- Especially in patients with known structural heart disease or other risk factors for toxicity, initiation or dose-adjustment of treatment with quinidine sulfate should generally be performed in a setting where facilities and personnel for monitoring and resuscitation are continuously available. Monitoring should be continued for two or three days after initiation of the regimen on which the patient will be discharged.
- Dosing Information
- Therapy with quinidine sulfate should begin with one tablet (300 mg; 249 mg of quinidine base) every eight to twelve hours.
- If this regimen is well tolerated, if the serum quinidine level is still well within the laboratory’s therapeutic range, and if the average time between arrhythmic episodes has not been satisfactorily increased, then the dose may be cautiously raised. The total daily dosage should be reduced if the QRS complex widens to 130% of its pre-treatment duration; the QTc interval widens to 130% of its pre-treatment duration and is then longer than 500 ms; P waves disappear; or the patient develops significant tachycardia, symptomatic bradycardia, or hypotension.
Suppression of Ventricular Arrhythmias
- Dosing regimens for the use of quinidine sulfate in suppressing life-threatening ventricular arrhythmias have not been adequately studied. Described regimens have generally been similar to the regimen described just above for the prophylaxis of symptomatic atrial fibrillation/flutter. Where possible, therapy should be guided by the results of programmed electrical stimulation and/or Holter monitoring with exercise.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Quinidine sulfate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Quinidine sulfate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness of the antiarrhythmic use of quinidine in pediatric patients have not been established in well-controlled clinical trials.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Quinidine sulfate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Quinidine sulfate in pediatric patients.
Contraindications
- Quinidine is contraindicated in patients who are known to be allergic to it, or who have developed thrombocytopenic purpura during prior therapy with quinidine or quinine.
- In the absence of a functioning artificial pacemaker, quinidine is also contraindicated in any patient whose cardiac rhythm is dependent upon a junctional or idioventricular pacemaker, including patients in complete atrioventricular block.
- Quinidine is also contraindicated in patients who, like those with myasthenia gravis, might be adversely affected by an anticholinergic agent.
Warnings
|
WARNINGS
See full prescribing information for complete Boxed Warning.
Mortality:
|
- Proarrhythmic Effects
- Like many other drugs (including all other Class Ia antiarrhythmics), quinidine prolongs the QT c interval, and this can lead to torsades de pointes, a life-threatening ventricular arrhythmia (see OVERDOSAGE). The risk of torsades is increased by bradycardia, hypokalemia, hypomagnesemia, or high serum levels of quinidine, but it may appear in the absence of any of these risk factors. The best predictor of this arrhythmia appears to be the length of the QTc interval, and quinidine should be used with extreme care in patients who have preexisting long-QT syndromes, who have histories of torsades de pointes of any cause, or who have previously responded to quinidine (or other drugs that prolong ventricular repolarization) with marked lengthening of the QTc interval. Estimation of the incidence of torsades in patients with therapeutic levels of quinidine is not possible from the available data.
- Other ventricular arrhythmias that have been reported with quinidine include frequent extrasystoles, ventricular tachycardia, ventricular flutter, and ventricular fibrillation.
- Paradoxical Increase in Ventricular Heart Rate in Atrial Flutter/Fibrillation
- When quinidine is administered to patients with atrial flutter/fibrillation, the desired pharmacologic reversion to sinus rhythm may (rarely) be preceded by a slowing of the atrial rate with a consequent increase in the rate of beats conducted to the ventricles. The resulting ventricular rate may be very high (greater than 200 beats per minute) and poorly tolerated. This hazard may be decreased if partial atrioventricular block is achieved prior to initiation of quinidine therapy, using conduction-reducing drugs such as digitalis, verapamil, diltiazem, or a β-receptor blocking agent.
- Exacerbated Bradycardia in Sick Sinus Syndrome
- In patients with the sick sinus syndrome, quinidine has been associated with marked sinus node depression and bradycardia.
- Pharmacokinetic Considerations
- Renal or hepatic dysfunction causes the elimination of quinidine to be slowed, while congestive heart failure causes a reduction in quinidine’s apparent volume of distribution. Any of these conditions can lead to quinidine toxicity if dosage is not appropriately reduced. In addition, interactions with coadministered drugs can alter the serum concentration and activity of quinidine, leading either to toxicity or to lack of efficacy if the dose of quinidine is not appropriately modified.
- Vagolysis
- Because quinidine opposes the atrial and A-V nodal effects of vagal stimulation, physical or pharmacological vagal maneuvers undertaken to terminate paroxysmal supraventricular tachycardia may be ineffective in patients receiving quinidine.
Precautions
- General
- All the precautions applying to regular quinidine therapy apply to this product. Hypersensitivity or anaphylactoid reactions to quinidine, although rare, should be considered, especially during the first weeks of therapy. Hospitalization for close clinical observation, electrocardiographic monitoring, and determination of serum quinidine levels are indicated when large doses of quinidine are used or with patients who present an increased risk.
- Laboratory Tests
- Periodic blood counts and liver and kidney function tests should be performed during long-term therapy; the drug should be discontinued if blood dyscrasias or evidence of hepatic or renal dysfunction occurs.
- Heart Block
- In patients without implanted pacemakers who are at high risk of complete atrioventricular block (e.g., those with digitalis intoxication, second-degree atrioventricular block, or severe intraventricular conduction defects), quinidine should be used only with caution.
Adverse Reactions
Clinical Trials Experience
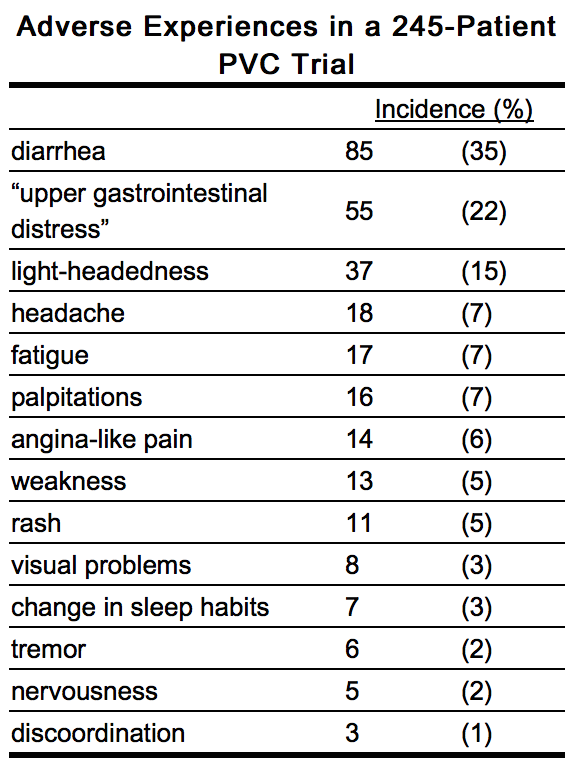
- Quinidine preparations have been used for many years, but there are only sparse data from which to estimate the incidence of various adverse reactions. The adverse reactions most frequently reported have consistently been gastrointestinal, including diarrhea, nausea, vomiting, and heartburn/esophagitis. In one study of 245 adult outpatients who received quinidine to suppress premature ventricular contractions, the incidences of reported adverse experiences were as shown in the table below.
- Vomiting and diarrhea can occur as isolated reactions to therapeutic levels of quinidine, but they also may be the first signs of cinchonism, a syndrome that also may include tinnitus, reversible high-frequency hearing loss, deafness, vertigo, blurred vision, diplopia, photophobia, headache, confusion, and delirium. Cinchonism is most often a sign of chronic quinidine toxicity, but it may appear in sensitive patients after a single moderate dose.
- A few cases of hepatotoxicity, including granulomatous hepatitis, have been reported in patients receiving quinidine. All of these have appeared during the first few weeks of therapy, and most (not all) have remitted once quinidine was withdrawn.
- Autoimmune and inflammatory syndromesassociated with quinidine therapy have included pneumonitis, fever, urticaria, flushing, exfoliative rash, bronchospasm, psoriasiform rash, pruritus and lymphadenopathy, hemolytic anemia, vasculitis, thrombocytopenic purpura, uveitis, angioedema, agranulocytosis, the sicca syndrome, arthralgia, myalgia, elevation in serum levels of skeletal-muscle enzymes, and a disorder resembling systemic lupus erythematosus.
- Convulsions, apprehension, and ataxia have been reported, but it is not clear that these were not simply the results of hypotension and consequent cerebral hypoperfusion. There are many reports of syncope.
- Acute psychotic reactions have been reported to follow the first dose of quinidine, but these reactions appear to be extremely rare.
- Other adverse reactions occasionally reported include depression, mydriasis, disturbed color perception, night blindness, scotomata, optic neuritis, visual field loss, photosensitivity, and abnormalities of pigmentation.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Quinidine sulfate in the drug label.
Drug Interactions
- Altered pharmacokinetics of quinidine
- Drugs that alkalinize the urine ( carbonic-anhydrase inhibitors, sodium bicarbonate, thiazide diuretics) reduce renal elimination of quinidine.
- By pharmacokinetic mechanisms that are not well understood, quinidine levels are increased by coadministration of amiodarone orcimetidine. Very rarely, and again by mechanisms not understood, quinidine levels are decreased by coadministration of nifedipine.
- Hepatic elimination of quinidine may be accelerated by coadministration of drugs ( phenobarbital, phenytoin, rifampin) that induce production of cytochrome P450IIIA4 (P450 3A4).
- Perhaps because of competition for the P450 3A4metabolic pathway, quinidine levels rise when ketoconazole is coadministered.
- Coadministration of propranolol usually does not affect quinidine pharmacokinetics, but in some studies the β-blocker appeared to cause increases in the peak serum levels of quinidine, decreases in quinidine’s volume of distribution, and decreases in total quinidine clearance. The effects (if any) of coadministration of other β-blockers on quinidine pharmacokinetics have not been adequately studied.
- Diltiazem significantly decreases the clearance and increases the t1/2 of quinidine, but quinidine does not alter the kinetics of diltiazem.
- Hepatic clearance of quinidine is significantly reduced during coadministration of verapamil, with corresponding increases in serum levels and half-life.
- Grapefruit juice inhibits P450 3A4-mediated metabolism of quinidine to 3-hydroxyquinidine. Although the clinical significance of this interaction is unknown, grapefruit juice should be avoided.
- The rate and extent of quinidine absorption may be affected by changes in dietary salt intake; a decrease in dietary salt intake may lead to an increase in plasma quinidine concentrations.
- Altered pharmacokinetics of other drugs
- Quinidine slows the elimination of digoxin and simultaneously reduces digoxin’s apparent volume of distribution. As a result, serum digoxin levels may be as much as doubled. When quinidine and digoxin are coadministered, digoxin doses usually need to be reduced. Serum levels of digitoxin are also raised when quinidine is coadministered, although the effect appears to be smaller.
- By a mechanism that is not understood, quinidine potentiates the anticoagulatory action of warfarin, and the anticoagulant dosage may need to be reduced.
- Cytochrome P 450 IID6 (P450 2D6) is an enzyme critical to the metabolism of many drugs, notably including mexiletine, somephenothiazines, and most polycyclic antidepressants. Constitutional deficiency of P450 2D6 is found in less than 1% of Orientals, in about 2% of American blacks, and in about 8% of American whites. Testing with debrisoquine is sometimes used to distinguish the P450 2D6-deficient “poor metabolizers” from the majority-phenotype “extensive metabolizers.”
- When drugs whose metabolism is P450 2D6-dependent are given to poor metabolizers, the serum levels achieved are higher, sometimes much higher, than the serum levels achieved when identical doses are given to extensive metabolizers. To obtain similar clinical benefit without toxicity, doses given to poor metabolizers may need to be greatly reduced. In the cases of prodrugs whose actions are actually mediated by P450 2D6-produced metabolites (for example, codeine and hydrocodone, whose analgesic and antitussive effects appear to be mediated by morphine and hydromorphone, respectively), it may not be possible to achieve the desired clinical benefits in poor metabolizers.
- Quinidine is not metabolized by P450 2D6, but therapeutic serum levels of quinidine inhibit the action of P450 2D6, effectively converting extensive metabolizers into poor metabolizers. Caution must be exercised whenever quinidine is prescribed together with drugs metabolized by P450 2D6.
- Perhaps by competing for pathways of renal clearance, coadministration of quinidine causes an increase in serum levels of procainamide. Serum levels of haloperidol are increased when quinidine is coadministered.
- Presumably because both drugs are metabolized by P450 3A4, coadministration of quinidine causes variable slowing of the metabolism ofnifedipine. Interactions with other dihydropyridine calcium-channel blockers have not been reported, but these agents (includingfelodipine, nicardipine, and nimodipine) are all dependent upon P450 3A4 for metabolism, so similar interactions with quinidine should be anticipated.
- Altered pharmacodynamics of other drugs
- Quinidine’s anticholinergic, vasodilating, and negative inotropic actions may be additive to those of other drugs with these effects, and antagonistic to those of drugs with cholinergic, vasoconstricting, and positive inotropic effects. For example, when quinidine and verapamilare coadministered in doses that are each well tolerated as monotherapy, hypotension attributable to additive peripheral α-blockade is sometimes reported.
- Quinidine potentiates the actions of depolarizing (succinylcholine, decamethonium) and nondepolarizing (d-tubocurarine, pancuronium)neuromuscular blocking agents. These phenomena are not well understood, but they are observed in animal models as well as in humans. In addition, in vitro addition of quinidine to the serum of pregnant women reduces the activity of pseudocholinesterase, an enzyme that is essential to the metabolism of succinylcholine.
- Non-interactions of quinidine with other drugs
- Quinidine has no clinically significant effect on the pharmacokinetics of diltiazem, flecainide, mephenytoin, metoprolol, propafenone, propranolol, quinine, timolol, or tocainide.
- Conversely, the pharmacokinetics of quinidine are not significantly affected by caffeine, ciprofloxacin, digoxin, felodipine, omeprazole, or quinine. Quinidine’s pharmacokinetics are also unaffected by cigarette smoking.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Animal reproductive studies have not been conducted with quinidine. There are no adequate and well-controlled studies in pregnant women. Quinidine should be given to a pregnant woman only if clearly needed.
- In one neonate whose mother had received quinidine throughout her pregnancy, the serum level of quinidine was equal to that of the mother, with no apparent ill effect. The level of quinidine in amniotic fluid was about three times higher than that found in serum.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Quinidine sulfate in women who are pregnant.
Labor and Delivery
- Quinine is said to be oxytocic in humans, but there are no adequate data as to quinidine’s effects (if any) on human labor and delivery.
Nursing Mothers
- Quinidine is present in human milk at levels slightly lower than those in maternal serum; a human infant ingesting such milk should (scaling directly by weight) be expected to develop serum quinidine levels at least an order of magnitude lower than those of the mother. On the other hand, the pharmacokinetics and pharmacodynamics of quinidine in human infants have not been adequately studied, and neonates’ reduced protein binding of quinidine may increase their risk of toxicity at low total serum levels. Administration of quinidine should (if possible) be avoided in lactating women who continue to nurse.
Pediatric Use
- In antimalarial trials, quinidine was as safe and effective in pediatric patients as in adults. Notwithstanding the known pharmacokinetic differences between the pediatric population and adults (see CLINICAL PHARMACOLOGY, Pharmacokinetics), pediatric patients in these trials received the same doses (on a mg/kg basis) as adults.
- Safety and effectiveness of the antiarrhythmic use of quinidine in pediatric patients have not been established in well-controlled clinical trials.
Geriatic Use
- Clinical studies of quinidine generally were not adequate to determine if significant safety or efficacy differences exist between elderly patients (65 years or older) and younger patients.
Quinidine clearance is apparently independent of age (see CLINICAL PHARMACOLOGY, Pharmacokinetics). However, renal or hepatic dysfunction causes the elimination of quinidine to be slowed (see WARNINGS, Pharmacokinetic Considerations), and since these conditions are more common in the elderly, appropriate dosing reductions should be considered in these individuals.
Gender
There is no FDA guidance on the use of Quinidine sulfate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Quinidine sulfate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Quinidine sulfate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Quinidine sulfate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Quinidine sulfate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Quinidine sulfate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Quinidine sulfate in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Quinidine sulfate in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Quinidine sulfate in the drug label.
Pharmacology
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Quinidine sulfate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Quinidine sulfate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Quinidine sulfate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Quinidine sulfate in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Quinidine sulfate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Quinidine sulfate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Quinidine sulfate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Before prescribing Quinidine Sulfate Extended-release Tablets as prophylaxis against recurrence of atrial fibrillation, the physician should inform the patient of the risks and benefits to be expected. Discussion should include the folliwng facts:
- The goal of therapy will be a reduction (probably not to zero) in the frequency of episodes of atrial fibrillation
- Reduced frequency of fibrillatory episodes may be expected, if achieved, to bring symptomatic benefit
- No data are available to show that reduced frequency of fibrillatory episodes will reduce the risks of irreversible harm through stroke or death
- Such data as are available suggest that treatment with quinidine sulfate is likely to increase the patient’s risk of death
Precautions with Alcohol
- Alcohol-Quinidine sulfate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Quinidine sulfate |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Quinidine sulfate |Label Name=Quinidine sulfate03.png
}}
{{#subobject:
|Label Page=Quinidine sulfate |Label Name=Quinidine sulfate04.png
}}