Procainamide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNINGS
See full prescribing information for complete Boxed Warning.
Mortality:
Blood Dyscrasias:
|
Overview
Procainamide is an antiarrhythmic that is FDA approved for the {{{indicationType}}} of ventricular arrhythmias, such as sustained ventricular tachycardia. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypotension and dysrhythmia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Ventricular Arrhythmias
- Procainamide is useful for arrhythmias which require immediate suppression and for maintenance of arrhythmia control. Intravenous therapy allows most rapid control of serious arrhythmias, including those following myocardial infarction; it should be carried out in circumstances where close observation and monitoring of the patient are possible, such as in hospital or emergency facilities. Intramuscular administration is less apt to produce temporary high plasma levels but therapeutic plasma levels are not obtained as rapidly as with intravenous administration. Oral procainamide dosage forms are preferable for less urgent arrhythmias as well as for long-term maintenance after initial parenteral PA therapy.
- Intramuscular administration may be used as an alternative to the oral route for patients with less threatening arrhythmias but who are nauseated or vomiting, who are ordered to receive nothing by mouth preoperatively, or who may have malabsorptive problems. An initial daily dose of 50 mg per kg body weight may be estimated. This amount should be divided into fractional doses of one-eighth to one-quarter to be injected intramuscularly every three to six hours until oral therapy is possible. If more than three injections are given, the physician may wish to assess patient factors such as age and renal function, clinical response and, if available, blood levels of PA and NAPA in adjusting further doses for that individual. For treatment of arrhythmias associated with anesthesia or surgical operation, the suggested dose is 100 to 500 mg by intramuscular injection.
- Intravenous administration of Procainamide should be done cautiously to avoid a possible hypotensive response. Initial arrhythmia control, under ECG monitoring, may usually be accomplished safely within a half-hour by either of the two methods which follows:
- Direct injection into a vein or into tubing of an established infusion line should be done slowly at a rate not to exceed 50 mg per minute. It is advisable to dilute either the 100 mg/mL or the 500 mg/mL concentrations of procainamide hydrochloride prior to intravenous injection to facilitate control of dosage rate. Doses of 100 mg may be administered every 5 minutes at this rate until the arrhythmia is suppressed or until 500 mg has been administered, after which it is advisable to wait 10 minutes or longer to allow for more distribution into tissues before resuming.
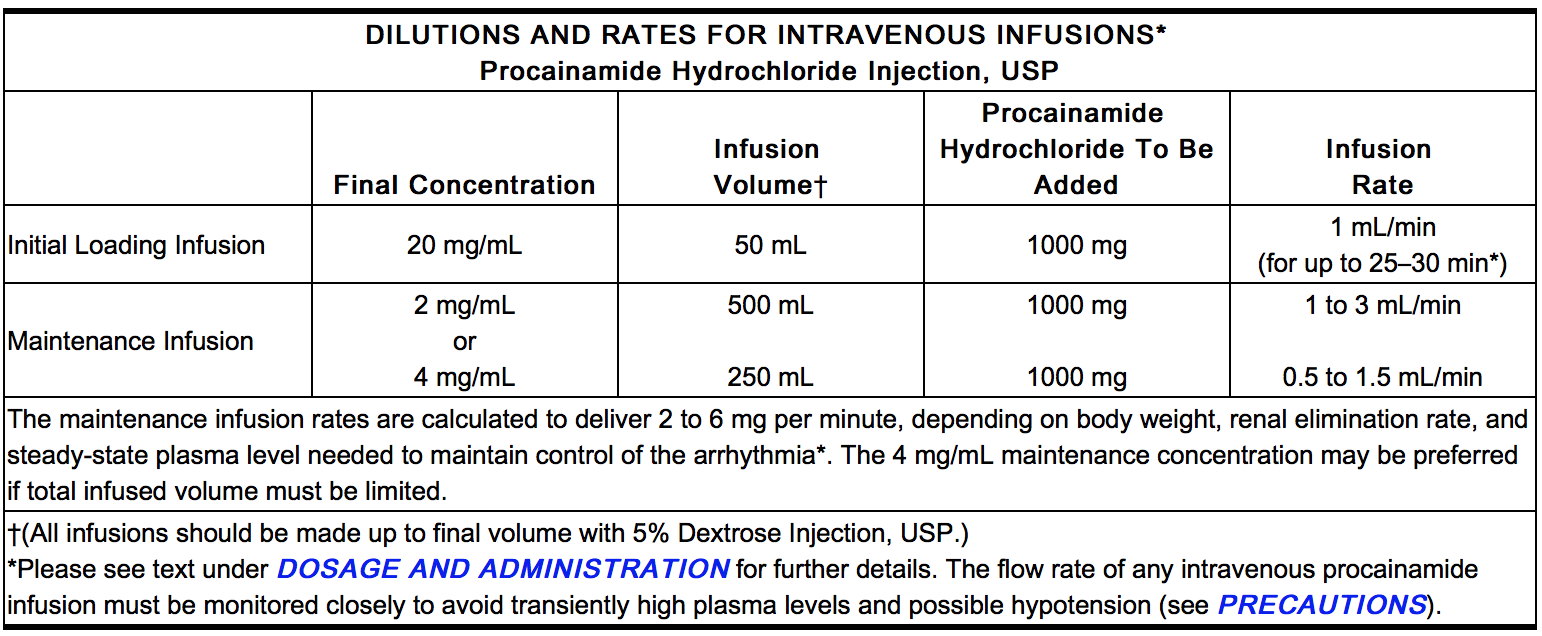
- Alternatively, a loading infusion containing 20 mg of Procainamide Hydrochloride per mL (1 g diluted to 50 mL with 5% Dextrose Injection, USP) may be administered at a constant rate of 1 mL per minute for 25 to 30 minutes to deliver 500 to 600 mg of PA. Some effects may be seen after infusion of the first 100 or 200 mg; it is unusual to require more than 600 mg to achieve satisfactory antiarrhythmic effects.
- The maximum advisable dosage to be given either by repeated bolus injections or such loading infusion is 1 g.
- To maintain therapeutic levels, a more dilute intravenous infusion at a concentration of 2 mg/mL is convenient (1000 mg procainamide HCl in 500 mL of 5% Dextrose Injection, USP), and may be administered at 1 to 3 mL/minute. If daily total fluid intake must be limited, a 4 mg/mL concentration (1 g of Procainamide Hydrochloride Injection in 250 mL of 5% Dextrose Injection, USP) administered at 0.5 to 1.5 mL/minute will deliver an equivalent 2 to 6 mg per minute. The amount needed in a given patient to maintain the therapeutic level should be assessed principally from the clinical response, and will depend upon the patient’s weight and age, renal elimination, hepatic acetylation rate, and cardiac status, but should be adjusted for each patient based upon close observation. A maintenance infusion rate of 50 mcg/min/kg body weight to a person with a normal renal PA elimination half-time of three hours may be expected to produce a plasma level of approximately 6.5 mcg/mL.
- Since the principal route for elimination of PA and NAPA is renal excretion, reduced excretion will prolong the half-life of elimination and lower the dose rate needed to maintain therapeutic levels. Advancing age reduces the renal excretion of PA and NAPA independently of reductions in creatinine clearance; compared to normal young adults, there is approximately 25 percent reduction at age 50 and 50 percent at age 75.
- Intravenous therapy should be terminated if persistent conduction disturbances or hypotension develop. As soon as the patient’s basic cardiac rhythm appears to be stabilized, oral antiarrhythmic maintenance therapy is preferable, if indicated and possible. A period of about three to four hours (one half-time for renal elimination, ordinarily) should elapse after the last intravenous dose before administering the first dose of Procainamide Hydrochloride tablets or capsules.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Stable Ventricular Tachycardia
- Developed by: ACC/AHA
- Class of Recommendation: Class IIa
- Strength of Evidence: Level B
- For patients who are stable with likely VT, IV antiarrhythmic drugs or elective cardioversion is the preferred treatment strategy. Procainamide can be administered at a rate of 20 to 50 mg/min until the arrhythmia is suppressed, hypotension ensues, QRS duration increases >50%, or the maximum dose of 17 mg/kg is given. Maintenance infusion is 1 to 4 mg/min. Procainamide should be avoided in patients with prolonged QT and congestive heart failure.[1]
Atrial Fibrillation
- Developed by: ACC/AHA
- Class of Recommendation: Class I
- Strength of Evidence: Level B
- In patients with pre-excited AF and rapid ventricular response who are hemodynamically stable, IV procainamide may be used to slow ventricular rate and convert to sinus rhythm.[2]
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Procainamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Procainamide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Procainamide in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Procainamide in pediatric patients.
Contraindications
- Condition1
Warnings
|
WARNINGS
See full prescribing information for complete Boxed Warning.
Mortality:
Blood Dyscrasias:
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Procainamide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Procainamide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Procainamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Procainamide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Procainamide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Procainamide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Procainamide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Procainamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Procainamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Procainamide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Procainamide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Procainamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Procainamide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
- Intramuscular
Monitoring
There is limited information regarding Monitoring of Procainamide in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Procainamide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Procainamide in the drug label.
Pharmacology
| |
Procainamide
| |
| Systematic (IUPAC) name | |
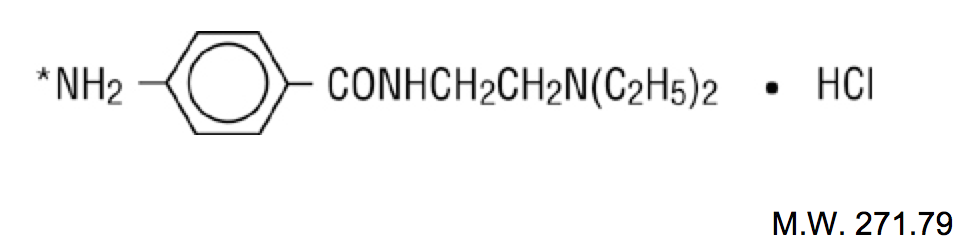
| 4-amino-N-(2-diethylaminoethyl) benzamide | |
| Identifiers | |
| CAS number | |
| ATC code | C01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 235.325 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 85% (oral) |
| Protein binding | 15 to 20% |
| Metabolism | Hepatic (CYP2D6-mediated) |
| Half life | ~2.5 to 4.5 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | IV, IM, oral |
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Procainamide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Procainamide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Procainamide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Procainamide in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Procainamide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Procainamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Procainamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Procainamide in the drug label.
Precautions with Alcohol
- Alcohol-Procainamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Procanbid®[3]
Look-Alike Drug Names
- Procanbid® — probenecid[4]
- Procanbid® — Procan SR®[4]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Neumar, Robert W. (2010-11-02). "Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care". Circulation. 122 (18 Suppl 3): –729-767. doi:10.1161/CIRCULATIONAHA.110.970988. ISSN 1524-4539. PMID 20956224. Unknown parameter
|coauthors=ignored (help) - ↑ January, Craig T. (2014-03-28). "2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society". Journal of the American College of Cardiology. doi:10.1016/j.jacc.2014.03.022. ISSN 1558-3597. PMID 24685669. Unknown parameter
|coauthors=ignored (help) - ↑ "PROCAINAMIDE HYDROCHLORIDE injection, solution".
- ↑ 4.0 4.1 "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Procainamide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Procainamide |Label Name=Procainamide11.png
}}
{{#subobject:
|Label Page=Procainamide |Label Name=Procainamide11.png
}}