Polio prevention: Difference between revisions
Joao Silva (talk | contribs) |
Joao Silva (talk | contribs) No edit summary |
||
| Line 6: | Line 6: | ||
Two [[polio vaccine]]s are used throughout the world to combat polio. Both [[vaccine]]s induce [[immunity]] to polio, efficiently blocking person-to-person transmission of wild [[poliovirus]], thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity). | Two [[polio vaccine]]s are used throughout the world to combat polio. Both [[vaccine]]s induce [[immunity]] to polio, efficiently blocking person-to-person transmission of wild [[poliovirus]], thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity). | ||
==Prevention == | ==Prevention== | ||
===Vaccine=== | |||
'''''Click [[Polio vaccine|here]] to read more about the polio vaccine.''''' | |||
Ever since its introduction, vaccination against poliomyelitis has been reducing the impact of the disease throughout the world. Two forms of the vaccine are available, the Inactivated Poliovirus Vaccine (IPV) and the Live-Attenuated Poliovirus Vaccine (OPV). | |||
<!-- | <!-- | ||
IPV | Both IPV and live-attenuated OPV have been used effectively for almost 50 years for controlling paralytic poliomyelitis. The introduc- tion of Salk IPV in 1955 led to an immediate and dramatic reduction in both epidemic and endemic poliomyelitis. However, after the vaccine became generally available, it was observed that as many as 17% of children with paralytic poliomyelitis had received three or more doses of IPV, suggesting that the original formulation lacked optimum potency.60 Meanwhile, live-attenuated OPV strains were developed by multiple passages of polioviruses in monkey kidney cell cultures and selection of mutants with low virulence for primates.61 Successful field trials of the Sabin OPV vaccine strains were carried out in the United States and many foreign countries from 1955 to 1959, and OPV was introduced for routine use in 1962 as separate monova- lent vaccines; the trivalent product became available in 1964. OPV was quickly accepted by the pediatric and public health community because of superior immunogenicity compared with the original IPV formula- tion, lower cost, ease of administration, spread of vaccine virus to unimmunized, susceptible persons, and induction of gastrointestinal immunity. These properties have made OPV the logical choice for continued use in developing countries. However, because OPV also carries the risk of causing rare cases of vaccine-associated paralytic poliomyelitis (VAPP), the United States and other developed countries have moved to exclusive use of a more potent IPV.62 | ||
--> | --> | ||
Two polio vaccines are used throughout the world to combat polio. Both vaccines induce immunity to polio, efficiently blocking person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity). | Two polio vaccines are used throughout the world to combat polio. Both vaccines induce immunity to polio, efficiently blocking person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity). | ||
| Line 32: | Line 33: | ||
{{Reflist|2}} | {{Reflist|2}} | ||
[[Category:Primary care]] | [[Category:Primary care]] | ||
[[Category:Disease]] | [[Category:Disease]] | ||
[[Category:Infectious disease]] | [[Category:Infectious disease]] | ||
{{WH}} | {{WH}} | ||
{{WS}} | {{WS}} | ||
Revision as of 18:47, 3 September 2014
|
Polio Microchapters |
|
Causes |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Polio prevention On the Web |
|
American Roentgen Ray Society Images of Polio prevention |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Two polio vaccines are used throughout the world to combat polio. Both vaccines induce immunity to polio, efficiently blocking person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity).
Prevention
Vaccine
Click here to read more about the polio vaccine. Ever since its introduction, vaccination against poliomyelitis has been reducing the impact of the disease throughout the world. Two forms of the vaccine are available, the Inactivated Poliovirus Vaccine (IPV) and the Live-Attenuated Poliovirus Vaccine (OPV).
Two polio vaccines are used throughout the world to combat polio. Both vaccines induce immunity to polio, efficiently blocking person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity).
Inactivated Poliovirus Vaccine
The first polio vaccine was developed in 1952 by Jonas Salk at the University of Pittsburgh, and announced to the world on April 12, 1955. The Salk vaccine, or inactivated poliovirus vaccine (IPV), is based on poliovirus grown in a type of monkey kidney tissue culture (Vero cell line), which is chemically-inactivated with formalin. After two doses of IPV, ninety percent or more of individuals develop protective antibody to all three serotypes of poliovirus, and at least 99% are immune to poliovirus following three doses. IPV is currently the vaccine of choice in most countries.
Oral Poliovirus Vaccine
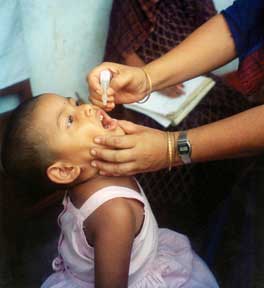
Eight years after Salk's success, Albert Sabin developed an oral polio vaccine (OPV) using live but weakened (attenuated) virus, produced by the repeated passage of the virus through non-human cells at sub-physiological temperatures. Human trials of Sabin's vaccine began in 1957 and it was licensed in 1962. The attenuated poliovirus in the Sabin vaccine replicates very efficiently in the gut, the primary site of wild poliovirus infection and replication, but the vaccine strain is unable to replicate efficiently within nervous system tissue. OPV produces excellent immunity in the intestine, which helps prevent infection with wild virus in areas where the virus is endemic. A single dose of oral polio vaccine produces immunity to all three poliovirus serotypes in approximately 50% of recipients. Three doses of live-attenuated OPV produce protective antibody to all three poliovirus types in more than 95% of recipients.
OPV consists of a mixture of live attenuated poliovirus strains of each of the three serotypes, selected by their ability to mimic the immune response following infection with wild polioviruses, but with a significantly reduced incidence of spreading to the central nervous system. Three or more spaced doses of OPV are required to generate adequate levels of seroconversion. The action of oral polio vaccine (OPV) is two-pronged. OPV produces antibodies in the blood ('humoral' or serum immunity) to all three types of poliovirus, and in the event of infection, this protects the individual against polio paralysis by preventing the spread of poliovirus to the nervous system. OPV strains also produce a local immune response in the lining ('mucous membrane') of the intestines - the primary site for poliovirus multiplication. The antibodies produced there inhibit the multiplication of subsequent infections of 'wild' (naturally occurring) virus. This intestinal immune response to OPV is probably a reason why mass campaigns with OPV have been shown to stop person-to-person transmission of wild poliovirus. In very rare cases, the administration of OPV results in vaccine-associated paralysis associated with a reversion of the vaccine strains to the more neurovirulent profile of wild poliovirus. In a few instances, such vaccine strains have become both neurovirulent and transmissible and have resulted in infectious poliomyelitis.
Passive Immunization
In 1950, William Hammon at the University of Pittsburgh purified the gamma globulin component of the blood plasma of polio survivors.[1] Hammon proposed that the gamma globulin, which contained antibodies to poliovirus, could be used to halt poliovirus infection, prevent disease, and reduce the severity of disease in other patients who had contracted polio. The results of a large clinical trial were promising; the gamma globulin was shown to be about 80% effective in preventing the development of paralytic poliomyelitis.[2] It was also shown to reduce the severity of the disease in patients that developed polio.[1] The gamma globulin approach was later deemed impractical for widespread use, however, due in large part to the limited supply of blood plasma, and the medical community turned its focus to the development of a polio vaccine.[3]
References
- ↑ 1.0 1.1 Hammon W (1955). "Passive immunization against poliomyelitis". Monogr Ser World Health Organ. 26: 357–70. PMID 14374581.
- ↑ Hammon W, Coriell L, Ludwig E; et al. (1954). "Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. 5. Reanalysis of results based on laboratory-confirmed cases". J Am Med Assoc. 156 (1): 21–7. PMID 13183798.
- ↑ Rinaldo C (2005). "Passive immunization against poliomyelitis: the Hammon gamma globulin field trials, 1951–1953". Am J Public Health. 95 (5): 790–9. PMID 15855454.