Phenylpropanolamine
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 2.1 to 3.4 hours. |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
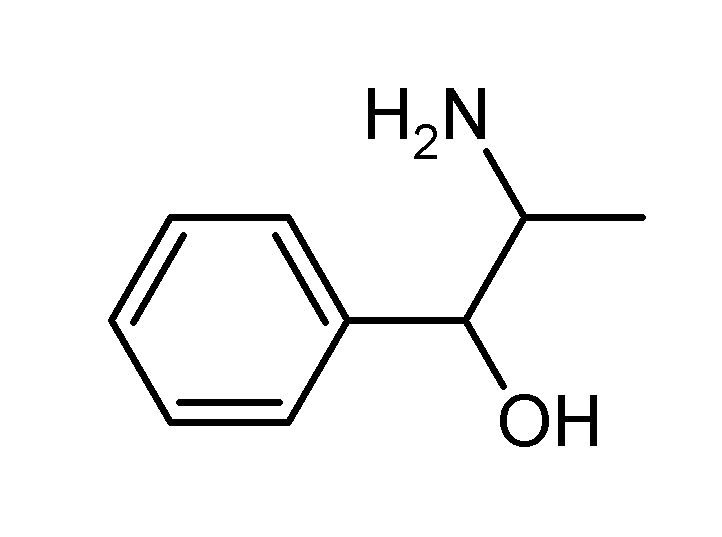
| Formula | C9H13NO |
| Molar mass | 151.206 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Phenylpropanolamine (PPA) is a drug of the phenethylamine family used as a decongestant and also as an appetite suppressant. In veterinary medicine, it is used to control urinary incontinence in dogs.
Chemistry
There are four optical isomers of phenylpropanolamine: d- and l-norephedrine, and d- and l-norpseudoephedrine. D-norpseudoephedrine is also known as cathine, and occurs naturally in the stimulant plant Catha edulis (khat). This isomer is commonly used in European medications described as "phenylpropanolamine", whereas in the United States a racemic mixture of d,l-norephedrine is usual.
Just as ephedrine is chemically reduced into methamphetamine, phenylpropanolamine can be chemically reduced into amphetamine. Molecularly, phenylpropanolamine is to ephedrine, just as amphetamine is to methamphetamine, and as cathinone is to methcathinone.
Phenylpropanolamine was also used for the illicit synthesis of other stimulant drugs such as phenmetrazine and 4-methylaminorex, and since phenylpropanolamine was withdrawn from use in humans in the early 2000s (although it is still sold for some veterinary applications) it is now much less available, and this in turn has meant that phenmetrazine and 4-methylaminorex have largely disappeared from the illicit market.
Phenylpropanolamine can be made from cathinone.
Side effects
A scientific study[1] found an increased risk of hemorrhagic stroke in women who used phenylpropanolamine, although it is not clear which isomer is to blame. A study at the Yale University School of Medicine in 1999 had produced similar results.[3] Reports of cases of hemorrhagic strokes in PPA users had been circulating since the 1970s.
A report from the Dept. of Psychiatry, F. Edward Hebert School of Medicine, Bethesda, Maryland in Pharmacopsychiatry[2] states:
- We have reviewed 37 cases (published in North America and Europe since 1960) that received diagnoses of acute mania, paranoid schizophrenia, and organic psychosis and that were attributed to PPA product ingestion. Of the 27 North American case reports, more reactions followed the ingestion of combination products than preparations containing PPA alone; more occurred after ingestion of over-the-counter products than those obtained by prescription or on-the-street; and more of the cases followed ingestion of recommended doses than overdoses.
- Failure to recognize PPA as an etiological agent in the onset of symptoms usually led to a diagnosis of schizophrenia or mania, lengthy hospitalization, and treatment with substantial doses of neuroleptics or lithium.
Legal control
In November 2000, the Food and Drug Administration (FDA) issued a public health advisory[3] against the use of the drug. In this advisory, the FDA requested that all drug companies discontinue marketing products containing phenylpropanolamine. The agency estimates that PPA caused between 200 and 500 strokes a year among 18-to-49-year-old users. In 2005 the FDA removed phenylpropanolamine from over-the-counter sale.[4] An item on the agenda of the 2000 Commission on Narcotic Drugs session called for including norephedrine in Table I of United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances[5]
Because of its potential use in methamphetamine manufacture, it is controlled by the Combat Methamphetamine Epidemic Act of 2005. It is still available for use in dogs.
See also
- phenethylamines
- Cathine
- ephedrine
- pseudoephedrine
- cathinone
- methcathinone
- amphetamine
- methamphetamine
- phenmetrazine
- 4-methylaminorex
References
- ↑ http://content.nejm.org/cgi/content/abstract/343/25/1826 Phenylpropanolamine and the Risk of Hemorrhagic Stroke], Kernan et al., 2000 N Engl J Med 343:1826-1832
- ↑ http://ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&list_uids=3060884 Psychiatric side effects attributed to phenylpropanolamine, Pharmacopsychiatry 1988 Jul; 21(4):171-81
- ↑ http://www.fda.gov/cder/drug/infopage/ppa/advisory.htm Food and Drug Administration Public Health Advisory, "Safety of Phenylpropanolamine", www.FDA.gov
- ↑ http://www.fda.gov/cder/drug/infopage/ppa/ FDA moves PPA from OTC
- ↑ http://www.unodc.org/unodc/en/document_1999-12-21_1.html Implementation of the international drug control treaties: changes in the scope of control of substances, Commission on Narcotic Drugs, Forty-third session, Vienna, 6-15 March 2000.
External links
- Phenylpropanolamine Information Page at www.FDA.gov
- A Dose of Denial: How drug makers sought to keep popular cold and diet remedies on store shelves after their own study linked them to strokes. By Kevin Sack and Alicia Mundy.Los Angeles Times, March 28, 2004.
- Doubt Is Their Product by David Michaels, Scientific American, June 2005
- Phenylpropanolamine by Richard Clapp, a case study at DefendingScience.org
- Widely Circulated Email About Phenylpropanolamine Description of widely circulated email using out-of-date information about Phenylpropanolamine.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Alcohols
- Alkaloids
- Amphetamines
- Anorectics
- Decongestants
- Sympathomimetic amines
- Withdrawn drugs
- DEA List I chemicals