Panobinostat
 | |
| Clinical data | |
|---|---|
| Trade names | Farydak |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 21%[1] |
| Protein binding | 90%[1] |
| Metabolism | CYP3A (40%), CYP2D6, CYP2C19[1] |
| Elimination half-life | 37 hours[1] |
| Excretion | Fecal (44–77%), renal (29–51%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
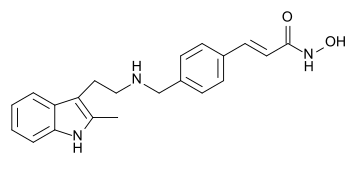
| Formula | C21H23N3O2 |
| Molar mass | 349.42622 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Panobinostat |
|
Articles |
|---|
|
Most recent articles on Panobinostat Most cited articles on Panobinostat |
|
Media |
|
Powerpoint slides on Panobinostat |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Panobinostat at Clinical Trials.gov Clinical Trials on Panobinostat at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Panobinostat
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Panobinostat Discussion groups on Panobinostat Patient Handouts on Panobinostat Directions to Hospitals Treating Panobinostat Risk calculators and risk factors for Panobinostat
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Panobinostat |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Panobinostat (LBH-589, trade name Farydak) is a drug developed by Novartis for the treatment of various cancers. It is a hydroxamic acid[2] and acts as a non-selective histone deacetylase inhibitor (pan-HDAC inhibitor).[3]
On 23 February 2015 it received FDA accelerated approval for use in patients with multiple myeloma who had received at least 2 previous treatments, including bortezomib and an immunomodulatory agent."[4] after clinical trials.[5]
Clinical trials
As of August 2012[update], it is being tested against Hodgkin's Lymphoma, cutaneous T cell lymphoma (CTCL)[6] and other types of malignant disease in Phase III clinical trials, against myelodysplastic syndromes, breast cancer and prostate cancer in Phase II trials, and against chronic myelomonocytic leukemia (CMML) in a Phase I trial.[7][8]
As of 2014[update] Panobinostat is being used in a Phase I/II clinical trial that aims at curing AIDS in patients on highly active antiretroviral therapy (HAART). In this technique, panobinostat is used to drive the HIV DNA out of the patient's DNA, in the expectation that the patient's immune system in combination with HAART will destroy it.[9][10] [11]
Preclinical studies
Panobinostat has been found to synergistically act with sirolimus to kill pancreatic cancer cells in the laboratory in a Mayo Clinic study. In the study, investigators found that this combination destroyed up to 65 percent of cultured pancreatic tumor cells. The finding is significant because the three cell lines studied were all resistant to the effects of chemotherapy – as are many pancreatic tumors.[12]
Panobinostat has also been found to significantly increase in vitro the survival of motor neuron (SMN) protein levels in cells of patients suffering from spinal muscular atrophy.[13]
Panobinostat was able to selectively target triple negative breast cancer (TNBC) cells by inducing hyperacetylation and cell cycle arrest at the G2-M DNA damage checkpoint; partially reversing the morphological changes characteristic of breast cancer cells.[14]
Panobinostat, along with other HDAC inhibitors, is also being studied for potential to induce virus HIV-1 expression in latently infected cells and disrupt latency. These resting cells are not recognized by the immune system as harboring the virus and do not respond to antiretroviral drugs.[15]
Mechanism of action
Panobinostat inhibits multiple histone deacetylase enzymes, a mechanism leading to apoptosis of malignant cells via multiple pathways.[2]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Panobinostat Package Insert
- ↑ 2.0 2.1 Revill, P; Mealy, N; Serradell, N; Bolos, J; Rosa, E (2007). "Panobinostat". Drugs of the Future. 32 (4): 315. doi:10.1358/dof.2007.032.04.1094476. ISSN 0377-8282.
- ↑ Table 3: Select epigenetic inhibitors in various stages of development from Template:Cite doi
- ↑ FDA.gov announcement about accelerated approval of panobinostat (Farydak)
- ↑ Panobinostat chemotherapy regimen for multiple myeloma (MM wiki)
- ↑ Clinical trial number NCT00425555 for "Study of Oral LBH589 in Adult Patients With Refractory Cutaneous T-Cell Lymphoma" at ClinicalTrials.gov
- ↑ ClinicalTrials.gov: LBH-589
- ↑ Prince, HM; M Bishton (2009). "Panobinostat (LBH589): a novel pan-deacetylase inhibitor with activity in T cell lymphoma". Hematology Meeting Reports. Parkville, Australia: Peter MacCallum Cancer Centre and University of Melbourne. 3 (1): 33–38.
- ↑ Simons, J (27 April 2013). "Scientists on brink of HIV cure". The Telegraph.
- ↑ Clinical trial number NCT01680094 for "Safety and Effect of The HDAC Inhibitor Panobinostat on HIV-1 Expression in Patients on Suppressive HAART (CLEAR)" at ClinicalTrials.gov
- ↑ Template:Cite doi
- ↑ Mayo Clinic Researchers Formulate Treatment Combination Lethal To Pancreatic Cancer Cells
- ↑ Garbes, L; Riessland, M; Hölker, I; Heller, R; Hauke, J; Tränkle, Ch; Coras, R; Blümcke, I; Hahnen, E; Wirth, B (2009). "LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate". Human Molecular Genetics. 18 (19): 3645–3658. doi:10.1093/hmg/ddp313. PMID 19584083.
- ↑ Tate, CR; Rhodes, LV; Segar, HC; Driver, JL; Pounder, FN; Burow, ME; Collins-Burow, BM (2012). "Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat". Breast Cancer Research. 14 (3).
- ↑ TA Rasmussen, et al. Comparison of HDAC inhibitors in clinical development: Effect on HIV production in latently infected cells and T-cell activation. Human Vaccines & Immunotherapeutics 9:5, 1-9, May 2013.
- Pages with script errors
- Articles with changed ChemSpider identifier
- Articles with changed KEGG identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Articles containing potentially dated statements from August 2012
- Articles with invalid date parameter in template
- All articles containing potentially dated statements
- Articles containing potentially dated statements from 2014
- Indoles
- Hydroxamic acids
- Histone deacetylase inhibitors
- Experimental cancer drugs