Norgestrel and Ethinyl estradiol: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag={{DB}} |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> |blackBoxWarningBody=<i><span style="color:#FF0000;">Condi...") |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{DB}} | |authorTag={{DB}} | ||
|genericName=Norgestrel and Ethinyl estradiol | |||
|aOrAn=an | |||
|drugClass=oral contraceptive agent | |||
|indication=contraception | |||
|adverseReactions=weight changes, abdominal pain, bloating symptom, nausea, vomiting, muscle cramps, headache, mood swings, amenorrhea, break-through bleeding, breast tenderness, discharge from nipple, disorder of menstruation, pain of breast, scanty vaginal bleeding | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
| Line 7: | Line 12: | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Norgestrel and Ethinyl estradiol in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Norgestrel and Ethinyl estradiol in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Norgestrel and Ethinyl estradiol in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Norgestrel and Ethinyl estradiol in pediatric patients. | ||
|drugBox=[[File:Norgestrel and Ethinyl estradiol image.png|600px|thumbnail|left]] | |||
{{clear}} | |||
|mechAction=Combination oral contraceptives act by suppression of gonadotrophins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which may reduce the likelihood of implantation). | |||
|structure= Low-Ogestrel ® Tablets (norgestrel and ethinyl estradiol tablets USP, 0.3 mg/0.03 mg) provide an oral contraceptive regimen consisting of 21 white tablets followed by 7 peach tablets. | |||
Each white tablet, for oral administration contains 0.3 mg of norgestrel and 0.03 mg ethinyl estradiol and the following inactive ingredients: croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, and povidone. | |||
Each inactive peach tablet, for oral administration, in the 28 day regimen contains the following inactive ingredients: anhydrous lactose, FD&C Yellow No. 6 Lake, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. | |||
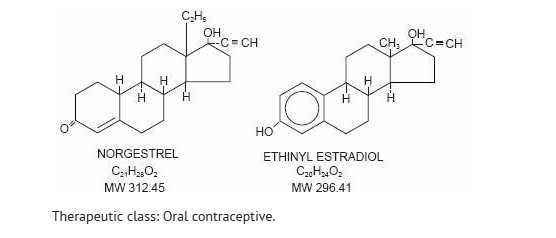
Norgestrel is a totally synthetic progestogen, insoluble in water, freely soluble in chloroform, sparingly soluble in alcohol with the chemical name (±)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one. Ethinyl estradiol is an estrogen, insoluble in water, soluble in alcohol, in chloroform, in ether, in vegetable oils, and in solutions of fixed alkali hydroxides with the chemical name 19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol. Their structural formulae follow: | |||
[[File:Norgestrel and Ethinyl estradiol structure.png|600px|thumbnail|left]] | |||
{{clear}} | |||
Therapeutic class: Oral contraceptive. | |||
|alcohol=Alcohol-Norgestrel and Ethinyl estradiol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Norgestrel and Ethinyl estradiol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 15:42, 30 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Norgestrel and Ethinyl estradiol is an oral contraceptive agent that is FDA approved for the {{{indicationType}}} of contraception. Common adverse reactions include weight changes, abdominal pain, bloating symptom, nausea, vomiting, muscle cramps, headache, mood swings, amenorrhea, break-through bleeding, breast tenderness, discharge from nipple, disorder of menstruation, pain of breast, scanty vaginal bleeding.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Norgestrel and Ethinyl estradiol FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Norgestrel and Ethinyl estradiol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Norgestrel and Ethinyl estradiol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Norgestrel and Ethinyl estradiol FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Norgestrel and Ethinyl estradiol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Norgestrel and Ethinyl estradiol in pediatric patients.
Contraindications
There is limited information regarding Norgestrel and Ethinyl estradiol Contraindications in the drug label.
Warnings
There is limited information regarding Norgestrel and Ethinyl estradiol Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Norgestrel and Ethinyl estradiol Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Norgestrel and Ethinyl estradiol Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Norgestrel and Ethinyl estradiol Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Norgestrel and Ethinyl estradiol in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Norgestrel and Ethinyl estradiol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Norgestrel and Ethinyl estradiol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol in geriatric settings.
Gender
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Norgestrel and Ethinyl estradiol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Norgestrel and Ethinyl estradiol in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Norgestrel and Ethinyl estradiol Administration in the drug label.
Monitoring
There is limited information regarding Norgestrel and Ethinyl estradiol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Norgestrel and Ethinyl estradiol and IV administrations.
Overdosage
There is limited information regarding Norgestrel and Ethinyl estradiol overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
Combination oral contraceptives act by suppression of gonadotrophins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which may reduce the likelihood of implantation).
Structure
Low-Ogestrel ® Tablets (norgestrel and ethinyl estradiol tablets USP, 0.3 mg/0.03 mg) provide an oral contraceptive regimen consisting of 21 white tablets followed by 7 peach tablets.
Each white tablet, for oral administration contains 0.3 mg of norgestrel and 0.03 mg ethinyl estradiol and the following inactive ingredients: croscarmellose sodium, lactose, magnesium stearate, microcrystalline cellulose, and povidone.
Each inactive peach tablet, for oral administration, in the 28 day regimen contains the following inactive ingredients: anhydrous lactose, FD&C Yellow No. 6 Lake, lactose monohydrate, magnesium stearate, and microcrystalline cellulose.
Norgestrel is a totally synthetic progestogen, insoluble in water, freely soluble in chloroform, sparingly soluble in alcohol with the chemical name (±)-13-Ethyl-17-hydroxy-18,19-dinor-17α-pregn-4-en-20-yn-3-one. Ethinyl estradiol is an estrogen, insoluble in water, soluble in alcohol, in chloroform, in ether, in vegetable oils, and in solutions of fixed alkali hydroxides with the chemical name 19-nor-17α-pregna-1,3,5(10)-trien-20-yne-3,17-diol. Their structural formulae follow:
Therapeutic class: Oral contraceptive.
Pharmacodynamics
There is limited information regarding Norgestrel and Ethinyl estradiol Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Norgestrel and Ethinyl estradiol Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Norgestrel and Ethinyl estradiol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Norgestrel and Ethinyl estradiol Clinical Studies in the drug label.
How Supplied
There is limited information regarding Norgestrel and Ethinyl estradiol How Supplied in the drug label.
Storage
There is limited information regarding Norgestrel and Ethinyl estradiol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Norgestrel and Ethinyl estradiol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Norgestrel and Ethinyl estradiol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Norgestrel and Ethinyl estradiol Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Norgestrel and Ethinyl estradiol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Norgestrel and Ethinyl estradiol Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Norgestrel and Ethinyl estradiol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.