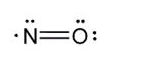Nitric oxide
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nitric oxide is a vasodilator that is FDA approved for the treatment of treatment of hypoxic respiratory failure. Common adverse reactions include hypotension, methemoglobinemia, hypoxemia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Nitric oxide FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nitric oxide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nitric oxide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Treatment of Hypoxic Respiratory Failure
- Nitric oxide is a vasodilator, which, in conjunction with ventilatory support and other appropriate agents, is indicated for the treatment of term and near-term (>34 weeks) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension, where it improves oxygenation and reduces the need for extracorporeal membrane oxygenation.
- Utilize additional therapies to maximize oxygen delivery with validated ventilation systems. In patients with collapsed alveoli, additional therapies might include surfactant and high-frequency oscillatory ventilation.
- The safety and effectiveness of nitric oxide have been established in a population receiving other therapies for hypoxic respiratory failure, including vasodilators, intravenous fluids, bicarbonate therapy, and mechanical ventilation. Different dose regimens for nitric oxide were used in the clinical studies.
- Monitor for PaO2, methemoglobin, and inspired NO2 during nitric oxide administration.
- To ensure safe and effective administration of nitric oxide to avoid adverse events associated with nitric oxide or NO2, administration of nitric oxide should only be performed by a health care professional who has completed and maintained training on the safe and effective use of a Nitric Oxide Delivery System provided by the manufacturer of the delivery system and the drug.
Dosage
- Term and near-term neonates with hypoxic respiratory failure
- The recommended dose of nitric oxide is 20 ppm. Treatment should be maintained up to 14 days or until the underlying oxygen desaturation has resolved and the neonate is ready to be weaned from nitric oxide therapy.
- As the risk of methemoglobinemia and elevated NO2 levels increases significantly when nitric oxide is administered at doses >20 ppm; doses above this level are not recommended.
Administration
- Methemoglobin should be measured within 4-8 hours after initiation of treatment with nitric oxide and periodically throughout treatment.
Nitric Oxide Delivery Systems
- Nitric oxide must be administered using the INOvent® Nitric Oxide Delivery Systems, which deliver operator-determined concentrations of nitric oxide in conjunction with a ventilator or breathing gas administration system after dilution with an oxygen/air mixture. A Nitric Oxide Delivery System includes a nitric oxide administration apparatus, a nitric oxide gas analyzer and a nitrogen dioxide gas analyzer. Failure to calibrate the Nitric Oxide Delivery System could result in under- or over- dosing of nitric oxide.
- To address potential power failure, keep available a backup battery power supply. To address potential system failure, keep available an independent reserve nitric oxide delivery system. Failure to transition to a reserve nitric oxide delivery system can result in abrupt or prolonged discontinuation of nitric oxide.
Training in Administration
- The user of nitric oxide and Nitric Oxide Delivery Systems must complete a comprehensive training program for health care professionals provided by the delivery system and drug manufacturers.
- Health professional staff that administers nitric oxide therapy have access to supplier-provided 24 hour/365 days per year technical support on the delivery and administration of nitric oxide.
Weaning and Discontinuation
- Abrupt discontinuation of nitric oxide may lead to increasing pulmonary artery pressure (PAP) and worsening oxygenation even in neonates with no apparent response to nitric oxide for inhalation. To wean nitric oxide, downtitrate in several steps, pausing several hours at each step to monitor for hypoxemia.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- Neonatal respiratory failure - Perinatal hypoxia - Pulmonary hypertension: neonates (greater than 34 wk gestation): 20 parts per million (ppm) via INHALATION for up to 14 days or until resolution of oxygen desaturation.
Non–Guideline-Supported Use
- Acute respiratory distress syndrome.
- Cardiovascular surgical procedure - Pulmonary hypertension
- Congestive heart failure.
- Diagnostic procedure, Pulmonary vasodilator testing.
- High altitude pulmonary edema.
- Primary pulmonary hypertension.
- Repair of congenital heart disease - Secondary pulmonary hypertension.
- Respiratory distress syndrome in the newborn, In preterm neonates in conjunction with mechanical ventilation and exogenous surfactant.
- Respiratory failure, pediatricView additional information.
- Right-sided heart failure, acute, After implantation of left ventricular assist device (LVAD) in patients with reversible pulmonary hypertension.
Contraindications
- It is contraindicated in the treatment of neonates known to be dependent on right-to-left shunting of blood.
Warnings
Rebound Pulmonary Hypertension Syndrome following Abrupt Discontinuation
- Abrupt discontinuation of nitric oxide may lead to worsening oxygenation and increasing pulmonary artery pressure, i.e., Rebound Pulmonary Hypertension Syndrome. Signs and symptoms of Rebound Pulmonary Hypertension Syndrome include hypoxemia, systemic hypotension, bradycardia, and decreased cardiac output. If Rebound Pulmonary Hypertension occurs, reinstate nitric oxide therapy immediately.
Hypoxemia from Methemoglobinemia
- Nitric oxide combines with hemoglobin to form methemoglobin, which does not transport oxygen. Methemoglobin levels increase with the dose of nitric oxide; it can take 8 hours or more before steady-state methemoglobin levels are attained. Monitor methemoglobin and adjust the dose of nitric oxide to optimize oxygenation.
- If methemoglobin levels do not resolve with decrease in dose or discontinuation of nitric oxide, additional therapy may be warranted to treat methemoglobinemia.
Airway Injury from Nitrogen Dioxide
- Nitrogen dioxide (NO2) forms in gas mixtures containing NO and O2. Nitrogen dioxide may cause airway inflammation and damage to lung tissues. If the concentration of NO2 in the breathing circuit exceeds 0.5 ppm, decrease the dose of nitric oxide.
- If there is an unexpected change in NO2 concentration, when measured in the breathing circuit, then the delivery system should be assessed in accordance with the Nitric Oxide Delivery System O&M Manual troubleshooting section, and the NO2 analyzer should be recalibrated. The dose of nitric oxide and/or FiO2 should be adjusted as appropriate.
Heart Failure
- Patients with left ventricular dysfunction treated with nitric oxide may experience pulmonary edema, increased pulmonary capillary wedge pressure, worsening of left ventricular dysfunction, systemic hypotension, bradycardia and cardiac arrest. Discontinue nitric oxide while providing symptomatic care.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The adverse reaction information from the clinical studies does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Clinical Trials Experience
- Controlled studies have included 325 patients on nitric oxide doses of 5 to 80 ppm and 251 patients on placebo. Total mortality in the pooled trials was 11% on placebo and 9% on nitric oxide, a result adequate to exclude nitric oxide mortality being more than 40% worse than placebo.
- In both the NINOS and CINRGI studies, the duration of hospitalization was similar in nitric oxide and placebo-treated groups.
- From all controlled studies, at least 6 months of follow-up is available for 278 patients who received nitric oxide and 212 patients who received placebo. Among these patients, there was no evidence of an adverse effect of treatment on the need for rehospitalization, special medical services, pulmonary disease, or neurological sequelae.
- In the NINOS study, treatment groups were similar with respect to the incidence and severity of intracranial hemorrhage, Grade IV hemorrhage, periventricular leukomalacia, cerebral infarction, seizures requiring anticonvulsant therapy, pulmonary hemorrhage, or gastrointestinal hemorrhage.
- In CINRGI, the only adverse reaction (>2% higher incidence on nitric oxide than on placebo) was hypotension (14% vs. 11%).
Postmarketing Experience
Accidental Exposure
- Based upon post-marketing experience, accidental exposure to nitric oxide for inhalation in hospital staff has been associated with chest discomfort, dizziness, dry throat, dyspnea, and headache.
Drug Interactions
- No formal drug-interaction studies have been performed, and a clinically significant interaction with other medications used in the treatment of hypoxic respiratory failure cannot be excluded based on the available data. nitric oxide has been administered with dopamine, dobutamine, steroids, surfactant, and high-frequency ventilation.
- Although there are no study data to evaluate the possibility, nitric oxide donor compounds, including sodium nitroprusside and nitroglycerin, may have an additive effect with nitric oxide on the risk of developing methemoglobinemia. An association between prilocaine and an increased risk of methemoglobinemia, particularly in infants, has specifically been described in a literature case report. This risk is present whether the drugs are administered as oral, parenteral, or topical formulations.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with nitric oxide. It is not known if nitric oxide can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. nitric oxide is not intended for adults.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nitric oxide in women who are pregnant.
Labor and Delivery
- The effect of nitric oxide on labor and delivery in humans is unknown.
Nursing Mothers
- Nitric oxide is not indicated for use in the adult population, including nursing mothers. It is not known whether nitric oxide is excreted in human milk.
Pediatric Use
- The safety and efficacy of nitric oxide for inhalation has been demonstrated in term and near-term neonates with hypoxic respiratory failure associated with evidence of pulmonary hypertension.
- Additional studies conducted in premature neonates for the prevention of bronchopulmonary dysplasia have not demonstrated substantial evidence of efficacy. No information about its effectiveness in other age populations is available.
Geriatic Use
- Nitric oxide is not indicated for use in the adult population.
Gender
There is no FDA guidance on the use of Nitric oxide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nitric oxide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nitric oxide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nitric oxide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nitric oxide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nitric oxide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Inhalational
Monitoring
There is limited information regarding Monitoring of Nitric oxide in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Nitric oxide in the drug label.
Overdosage
- Overdosage with nitric oxide will be manifest by elevations in methemoglobin and pulmonary toxicities associated with inspired NO2. Elevated NO2 may cause acute lung injury. Elevations in methemoglobin reduce the oxygen delivery capacity of the circulation. In clinical studies, NO2 levels >3 ppm or methemoglobin levels >7% were treated by reducing the dose of, or discontinuing, nitric oxide.
- Methemoglobinemia that does not resolve after reduction or discontinuation of therapy can be treated with intravenous vitamin C, intravenous methylene blue, or blood transfusion, based upon the clinical situation.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox 3DMetTemplate:Chembox ECNumberTemplate:Chembox E numberTemplate:Chembox GmelinTemplate:Chembox RTECSTemplate:Chembox UNNumberTemplate:Chembox AppearanceTemplate:Chembox DensityTemplate:Chembox MeltingPtTemplate:Chembox BoilingPtTemplate:Chembox SolubilityInWaterTemplate:Chembox RefractIndexTemplate:Chembox StructureTemplate:Chembox ThermochemistryTemplate:Chembox PharmacologyTemplate:Chembox EUClassTemplate:Chembox RPhrasesTemplate:Chembox SPhrasesTemplate:Chembox NFPATemplate:Chembox Supplement| Template:Chembox header2 | Nitric oxide | |
|---|---|
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| NO | |
| Molar mass | 30.01 g·mol−1 |
| Hazards | |
| Related compounds | |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
- Nitric oxide is a compound produced by many cells of the body. It relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3',5'-monophosphate, which then leads to vasodilation. When inhaled, nitric oxide selectively dilates the pulmonary vasculature, and because of efficient scavenging by hemoglobin, has minimal effect on the systemic vasculature.
- Nitric oxide appears to increase the partial pressure of arterial oxygen (PaO2) by dilating pulmonary vessels in better ventilated areas of the lung, redistributing pulmonary blood flow away from lung regions with low ventilation/perfusion (V/Q) ratios toward regions with normal ratios.
Structure
- Nitric oxide (nitric oxide gas) is a drug administered by inhalation. Nitric oxide, the active substance in nitric oxide, is a pulmonary vasodilator.
- Nitric oxide is a gaseous blend of nitric oxide and nitrogen (0.08% and 99.92%, respectively for 800 ppm; 0.01% and 99.99%, respectively for 100 ppm). nitric oxide is supplied in aluminum cylinders as a compressed gas under high pressure (2000 pounds per square inch gauge [psig]).
- The structural formula of nitric oxide (NO) is shown below:
Pharmacodynamics
Effects on Pulmonary Vascular Tone in PPHN
- Persistent pulmonary hypertension of the newborn (PPHN) occurs as a primary developmental defect or as a condition secondary to other diseases such as meconium aspiration syndrome (MAS), pneumonia, sepsis, hyaline membrane disease, congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia. In these states, pulmonary vascular resistance (PVR) is high, which results in hypoxemia secondary to right-to-left shunting of blood through the patent ductus arteriosus and foramen ovale. In neonates with PPHN, nitric oxide improves oxygenation (as indicated by significant increases in PaO2).
Pharmacokinetics
Effects on Pulmonary Vascular Tone in PPHN
- Persistent pulmonary hypertension of the newborn (PPHN) occurs as a primary developmental defect or as a condition secondary to other diseases such as meconium aspiration syndrome (MAS), pneumonia, sepsis, hyaline membrane disease, congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia. In these states, pulmonary vascular resistance (PVR) is high, which results in hypoxemia secondary to right-to-left shunting of blood through the patent ductus arteriosus and foramen ovale. In neonates with PPHN, nitric oxide improves oxygenation (as indicated by significant increases in PaO2).
Pharmacokinetics
- The pharmacokinetics of nitric oxide has been studied in adults.
Uptake and Distribution
- Nitric oxide is absorbed systemically after inhalation. Most of it traverses the pulmonary capillary bed where it combines with hemoglobin that is 60% to 100% oxygen-saturated. At this level of oxygen saturation, nitric oxide combines predominantly with oxyhemoglobin to produce methemoglobin and nitrate. At low oxygen saturation, nitric oxide can combine with deoxyhemoglobin to transiently form nitrosylhemoglobin, which is converted to nitrogen oxides and methemoglobin upon exposure to oxygen. Within the pulmonary system, nitric oxide can combine with oxygen and water to produce nitrogen dioxide and nitrite, respectively, which interact with oxyhemoglobin to produce methemoglobin and nitrate. Thus, the end products of nitric oxide that enter the systemic circulation are predominantly methemoglobin and nitrate.
Metabolism
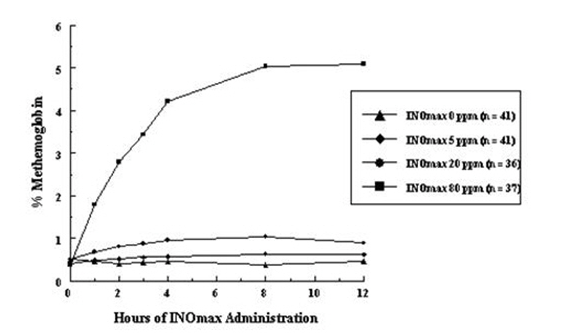
- Methemoglobin disposition has been investigated as a function of time and nitric oxide exposure concentration in neonates with respiratory failure. The methemoglobin (MetHb) concentration-time profiles during the first 12 hours of exposure to 0, 5, 20, and 80 ppm nitric oxide are shown in Figure 1.
- Methemoglobin concentrations increased during the first 8 hours of nitric oxide exposure. The mean methemoglobin level remained below 1% in the placebo group and in the 5 ppm and 20 ppm nitric oxide groups, but reached approximately 5% in the 80 ppm nitric oxide group. Methemoglobin levels >7% were attained only in patients receiving 80 ppm, where they comprised 35% of the group. The average time to reach peak methemoglobin was 10 ± 9 (SD) hours (median, 8 hours) in these 13 patients, but one patient did not exceed 7% until 40 hours.
Elimination
- Nitrate has been identified as the predominant nitric oxide metabolite excreted in the urine, accounting for >70% of the nitric oxide dose inhaled. Nitrate is cleared from the plasma by the kidney at rates approaching the rate of glomerular filtration.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No evidence of a carcinogenic effect was apparent, at inhalation exposures up to the recommended dose (20 ppm), in rats for 20 hr/day for up to two years. Higher exposures have not been investigated.
- Nitric oxide has demonstrated genotoxicity in Salmonella (Ames Test), human lymphocytes, and after in vivo exposure in rats. There are no animal or human studies to evaluate nitric oxide for effects on fertility.
Clinical Studies
Treatment of Hypoxic Respiratory Failure (HRF)
- The efficacy of nitric oxide has been investigated in term and near-term newborns with hypoxic respiratory failure resulting from a variety of etiologies. Inhalation of nitric oxide reduces the oxygenation index (OI= mean airway pressure in cm H2O × fraction of inspired oxygen concentration [FiO2]× 100 divided by systemic arterial concentration in mm Hg [PaO2]) and increases PaO2.
NINOS Study
- The Neonatal Inhaled Nitric Oxide Study (NINOS) was a double-blind, randomized, placebo-controlled, multicenter trial in 235 neonates with hypoxic respiratory failure.
- The objective of the study was to determine whether inhaled nitric oxide would reduce the occurrence of death and/or initiation of extracorporeal membrane oxygenation (ECMO) in a prospectively defined cohort of term or near-term neonates with hypoxic respiratory failure unresponsive to conventional therapy. Hypoxic respiratory failure was caused by meconium aspiration syndrome (MAS; 49%), pneumonia/sepsis (21%), idiopathic primary pulmonary hypertension of the newborn (PPHN; 17%), or respiratory distress syndrome (RDS; 11%).
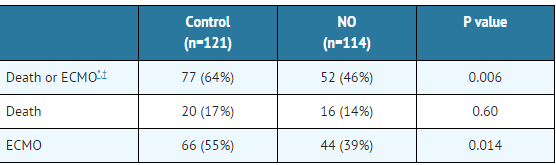
- Infants ≤14 days of age (mean, 1.7 days) with a mean PaO2 of 46 mm Hg and a mean oxygenation index (OI) of 43 cm H2O / mm Hg were initially randomized to receive 100% O2 with (n=114) or without (n=121) 20 ppm nitric oxide for up to 14 days. Response to study drug was defined as a change from baseline in PaO2 30 minutes after starting treatment (full response = >20 mm Hg, partial = 10–20 mm Hg, no response = <10 mm Hg). Neonates with a less than full response were evaluated for a response to 80 ppm nitric oxide or control gas. The primary results from the NINOS study are presented in Table 1.
- Although the incidence of death by 120 days of age was similar in both groups (NO, 14%; control, 17%), significantly fewer infants in the nitric oxide group required ECMO compared with controls (39% vs. 55%, p = 0.014). The combined incidence of death and/or initiation of ECMO showed a significant advantage for the nitric oxide treated group (46% vs. 64%, p = 0.006). The nitric oxide group also had significantly greater increases in PaO2 and greater decreases in the OI and the alveolar-arterial oxygen gradient than the control group (p<0.001 for all parameters).
- Significantly more patients had at least a partial response to the initial administration of study drug in the nitric oxide group (66%) than the control group (26%, p<0.001). Of the 125 infants who did not respond to 20 ppm nitric oxide or control, similar percentages of NO-treated (18%) and control (20%) patients had at least a partial response to 80 ppm nitric oxide for inhalation or control drug, suggesting a lack of additional benefit for the higher dose of nitric oxide.
- No infant had study drug discontinued for toxicity. Inhaled nitric oxide had no detectable effect on mortality. The adverse events collected in the NINOS trial occurred at similar incidence rates in both treatment groups.
- Follow-up exams were performed at 18–24 months for the infants enrolled in this trial. In the infants with available follow-up, the two treatment groups were similar with respect to their mental, motor, audiologic, or neurologic evaluations.
CINRGI Study
- This study was a double-blind, randomized, placebo-controlled, multicenter trial of 186 term and near-term neonates with pulmonary hypertension and hypoxic respiratory failure.
- The primary objective of the study was to determine whether nitric oxide would reduce the receipt of ECMO in these patients. Hypoxic respiratory failure was caused by MAS (35%), idiopathic PPHN (30%), pneumonia/sepsis (24%), or RDS (8%).
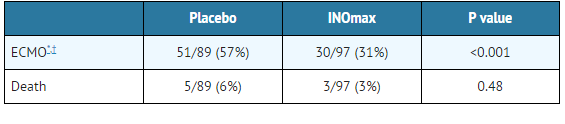
- Patients with a mean PaO2 of 54 mm Hg and a mean OI of 44 cm H2O / mm Hg were randomly assigned to receive either 20 ppm nitric oxide (n=97) or nitrogen gas (placebo; n=89) in addition to their ventilatory support. Patients who exhibited a PaO2 >60 mm Hg and a pH < 7.55 were weaned to 5 ppm nitric oxide or placebo. The primary results from the CINRGI study are presented in Table 2.
- Significantly fewer neonates in the nitric oxide group required ECMO compared to the control group (31% vs. 57%, p<0.001). While the number of deaths were similar in both groups (nitric oxide, 3%; placebo, 6%), the combined incidence of death and/or receipt of ECMO was decreased in the nitric oxide group (33% vs. 58%, p<0.001).
- In addition, the nitric oxide group had significantly improved oxygenation as measured by PaO2, OI, and alveolar-arterial gradient (p<0.001 for all parameters). Of the 97 patients treated with nitric oxide, 2 (2%) were withdrawn from study drug due to methemoglobin levels >4%. The frequency and number of adverse events reported were similar in the two study groups.
- In clinical trials, reduction in the need for ECMO has not been demonstrated with the use of inhaled nitric oxide in neonates with congenital diaphragmatic hernia (CDH).
Ineffective in Adult Respiratory Distress Syndrome (ARDS)
- In a randomized, double-blind, parallel, multicenter study, 385 patients with adult respiratory distress syndrome (ARDS) associated with pneumonia (46%), surgery (33%), multiple trauma (26%), aspiration (23%), pulmonary contusion (18%), and other causes, with PaO2/FiO2 <250 mm Hg despite optimal oxygenation and ventilation, received placebo (n=193) or nitric oxide (n=192), 5 ppm, for 4 hours to 28 days or until weaned because of improvements in oxygenation.
- Despite acute improvements in oxygenation, there was no effect of nitric oxide on the primary endpoint of days alive and off ventilator support. These results were consistent with outcome data from a smaller dose ranging study of nitric oxide (1.25 to 80 ppm). Nitric oxide is not indicated for use in ARDS.
Ineffective in Prevention of Bronchopulmonary Dysplasia (BPD)
- The safety and efficacy of nitric oxide for the prevention of chronic lung disease [bronchopulmonary dysplasia, (BPD)] in neonates ≤ 34 weeks gestational age requiring respiratory support has been studied in three large, multi-center, double-blind, placebo-controlled clinical trials in a total of 2,149 preterm infants. Of these, 1,068 received placebo, and 1,081 received inhaled nitric oxide at doses ranging from 5-20 ppm, for treatment periods of 7-24 days duration.
- The primary endpoint for these studies was alive and without BPD at 36 weeks postmenstrual age (PMA). The need for supplemental oxygen at 36 weeks PMA served as a surrogate endpoint for the presence of BPD.
- Overall, efficacy for the prevention of bronchopulmonary dysplasia in preterm infants was not established.
- There were no meaningful differences between treatment groups with regard to deaths, methemoglobin levels, or adverse events commonly observed in premature infants, including intraventricular hemorrhage, patent ductus arteriosus, pulmonary hemorrhage, and retinopathy of prematurity. The use of nitric oxide for prevention of BPD in preterm neonates ≤ 34 weeks gestational age is not indicated.
How Supplied
Storage
- Store at 25°C (77°F) with excursions permitted between 15–30°C (59–86°F) [see USP Controlled Room Temperature].
- All regulations concerning handling of pressure vessels must be followed.
- Protect the cylinders from shocks, falls, oxidizing and flammable materials, moisture, and sources of heat or ignition.
- The cylinders should be appropriately transported to protect from risks of shocks and falls.
Occupational Exposure
- The exposure limit set by the Occupational Safety and Health Administration (OSHA) for nitric oxide is 25 ppm, and for NO2 the limit is 5 ppm.
Images
Drug Images
{{#ask: Page Name::Nitric oxide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Nitric oxide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Nitric oxide in the drug label.
Precautions with Alcohol
- Alcohol-Nitric oxide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- INOMAX ®[1]
Look-Alike Drug Names
There is limited information regarding Nitric oxide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Nitric oxide |Label Name=Nitric oxide 6.jpg
}}
{{#subobject:
|Label Page=Nitric oxide |Label Name=Nitric oxide 7.jpg
}}