Monobenzone: Difference between revisions
No edit summary |
m (Protected "Monobenzone": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (9 intermediate revisions by one other user not shown) | |||
| Line 5: | Line 5: | ||
|drugClass=hypopigmentation agent | |drugClass=hypopigmentation agent | ||
|indicationType=treatment | |indicationType=treatment | ||
|indication=for final depigmentation in extensive | |indication=for final depigmentation in extensive [[vitiligo]] | ||
|adverseReactions= | |adverseReactions=achromia of [[skin]], permanent, burning sensation, [[dermatitis]], skin irritation, transient | ||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| Line 15: | Line 15: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult=Benoquin Cream 20% is indicated for final depigmentation in extensive | |fdaLIADAdult=* Benoquin Cream 20% is indicated for final depigmentation in extensive [[vitiligo]]. | ||
* Benoquin Cream 20% is applied topically to permanently depigment normal skin surrounding vitiliginous lesions in patients with disseminated (greater than 50 percent of body surface area) idiopathic [[vitiligo]]. | |||
Benoquin Cream 20% is applied topically to permanently depigment normal skin surrounding vitiliginous lesions in patients with disseminated (greater than 50 percent of body surface area) idiopathic vitiligo. | * Benoquin Cream 20% is not recommended in freckling; hyperpigmentation caused by photosensitization following the use of certain perfumes (berlock dermatitis); melasma (chloasma) of [[pregnancy]]; or hyperpigmentation resulting from inflammation of the [[skin]]. Benoquin Cream 20% is not effective for the treatment of [[cafe-au-lait spots]], pigmented nevi, [[malignant melanoma]] or pigmentation resulting from pigments other than [[melanin]] (e.g.: bile, silver, or artificial pigments). | ||
* A thin layer of Benoquin Cream 20% should be applied and rubbed into the pigmented area two or three times daily, or as directed by physician. Prolonged exposure to sunlight should be avoided during treatment with Benoquin Cream 20%, or a sunscreen should be used. | |||
Benoquin Cream 20% is not recommended in freckling; hyperpigmentation caused by photosensitization following the use of certain perfumes (berlock dermatitis); melasma (chloasma) of pregnancy; or hyperpigmentation resulting from inflammation of the skin. Benoquin Cream 20% is not effective for the treatment of cafe-au-lait spots, pigmented nevi, malignant melanoma or pigmentation resulting from pigments other than melanin (e.g.: bile, silver, or artificial pigments). | * Depigmentation is usually accomplished after one to four months of Benoquin Cream 20% treatment. If satisfactory results are not obtained after four months of Benoquin Cream 20% treatment, the drug should be discontinued. When the desired degree of depigmentation is obtained, Benoquin Cream 20% should be applied only as often as needed to maintain depigmentation (usually only two times weekly). | ||
A thin layer of Benoquin Cream 20% should be applied and rubbed into the pigmented area two or three times daily, or as directed by physician. Prolonged exposure to sunlight should be avoided during treatment with Benoquin Cream 20%, or a sunscreen should be used. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
Depigmentation is usually accomplished after one to four months of Benoquin Cream 20% treatment. If satisfactory results are not obtained after four months of Benoquin Cream 20% treatment, the drug should be discontinued. When the desired degree of depigmentation is obtained, Benoquin Cream 20% should be applied only as often as needed to maintain depigmentation (usually only two times weekly). | |||
|offLabelAdultGuideSupport= | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | <!--Non–Guideline-Supported Use (Adult)--> | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | <!--Pediatric Indications and Dosage--> | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|fdaLIADPed= | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
<!--Guideline-Supported Use (Pediatric)--> | <!--Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport= | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* Benoquin Cream 20% contains a potent depigmenting agent and is not a cosmetic skin bleach. Use of Benoquin Cream 20% is contraindicated in any conditions other than disseminated vitiligo. Benoquin Cream 20% frequently produces irreversible depigmentation, and it must not be used as a substitute for hydroquinone. | |contraindications=* Benoquin Cream 20% contains a potent depigmenting agent and is not a cosmetic skin bleach. Use of Benoquin Cream 20% is contraindicated in any conditions other than disseminated [[vitiligo]]. Benoquin Cream 20% frequently produces irreversible depigmentation, and it must not be used as a substitute for hydroquinone. | ||
* Benoquin Cream 20% is also contraindicated in individuals with a history of sensitivity or allergic reactions to this product, or any of its ingredients. | |||
Benoquin Cream 20% is also contraindicated in individuals with a history of sensitivity or allergic reactions to this product, or any of its ingredients. | |warnings=* Benoquin Cream 20% is a potent depigmenting agent, not a mild cosmetic bleach. Do not use except for final depigmentation in extensive [[vitiligo]]. | ||
|warnings=* Benoquin Cream 20% is a potent depigmenting agent, not a mild cosmetic bleach. Do not use except for final depigmentation in extensive vitiligo. | * Keep this, and all medications out of the reach of children. In case of accidental ingestion, call a physician or a Poison Control Center immediately. | ||
Keep this, and all medications out of the reach of children. In case of accidental ingestion, call a physician or a Poison Control Center immediately. | |||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | ||
|postmarketing=* Mild, transient skin irritation and sensitization, including erythematous and eczematous reactions have occurred following topical application of Benoquin Cream 20%. Although those reactions are usually transient, treatment with Benoquin Cream 20% should be discontinued if irritation, a burning sensation, or dermatitis occur. Areas of normal skin distant to the site of Benoquin Cream 20% application frequently have become depigmented, and irregular, excessive, unsightly, and frequently permanent depigmentation has occurred. | |||
|drugInteractions=<!--Use in Specific Populations--> | |||
|postmarketing=Mild, transient skin irritation and sensitization, including erythematous and eczematous reactions have occurred following topical application of Benoquin Cream 20%. Although those reactions are usually transient, treatment with Benoquin Cream 20% should be discontinued if irritation, a burning sensation, or dermatitis occur. Areas of normal skin distant to the site of Benoquin Cream 20% application frequently have become depigmented, and irregular, excessive, unsightly, and frequently permanent depigmentation has occurred. | |FDAPregCat=C | ||
|drugInteractions= | |useInPregnancyFDA=* Animal reproduction studies have not been conducted with Benoquin Cream 20%. It is also not known whether Benoquin Cream 20% can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. Benoquin Cream 20% should be given to a pregnant woman only if clearly needed. | ||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA=* | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | ||
|useInNursing= | |useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Benoquin Cream 20% is administered to a nursing woman. | ||
|useInPed= | |useInPed=The safety and effectiveness of Benoquin Cream 20% in pediatric patients below the age of 12 years have not been established. | ||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
| Line 77: | Line 63: | ||
<!--Administration and Monitoring--> | <!--Administration and Monitoring--> | ||
|administration=* | |administration=* Topical | ||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | ||
| Line 88: | Line 72: | ||
<!--Overdosage--> | <!--Overdosage--> | ||
|drugBox=<!--Mechanism of Action--> | |drugBox=<!--Mechanism of Action--> | ||
|mechAction=* | |mechAction=* Benoquin Cream 20% is a depigmenting agent whose mechanism of action is not fully understood. | ||
* The topical application of monobenzone in animals, increases the excretion of melanin from the [[melanocytes]]. The same action is thought to be responsible for the depigmenting effect of the drug in humans. Monobenzone may cause destruction of [[melanocytes]] and permanent [[depigmentation]]. This effect is erratic and may take one to four months to occur while existing [[melanin]] is lost with normal sloughing of the stratum corneum. Hyperpigmented [[skin]] appears to fade more rapidly than does normal [[skin]], and exposure to sunlight reduces the depigmenting effect of the drug. The histology of the skin after depigmentation with topical monobenzone is the same as that seen in [[vitiligo]]; the epidermis is normal except for the absence of identifiable [[melanocytes]]. | |||
<!--Structure--> | <!--Structure--> | ||
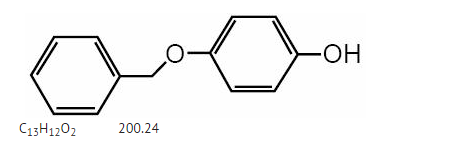
|structure=* | |structure=* Monobenzone is the monobenzyl ether of hydroquinone. Monobenzone occurs as a white, almost tasteless crystalline powder, soluble in alcohol and practically insoluble in water. | ||
* Chemically, monobenzone is designated as p-(benzyloxy) phenol; the empirical formula is C13H12O2; molecular weight 200.24. The structural formula is: | |||
: [[File: | : [[File:Meb 01 Structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
* Each gram of Benoquin Cream contains 200 mg of monobenzone USP, in a water-washable base consisting of purified water USP, cetyl alcohol NF, propylene glycol USP, sodium lauryl sulfate NF and white wax NF. | |||
<!--Pharmacodynamics--> | <!--Pharmacodynamics--> | ||
| Line 126: | Line 95: | ||
<!--How Supplied--> | <!--How Supplied--> | ||
|howSupplied=* | |howSupplied=* Benoquin Cream 20% in 1 1/4 oz. tubes (35.4 g) | ||
:* (NDC 0187-0380-34). | |||
|storage=* Benoquin Cream 20% should be stored at 25°C (77°F); | |||
:* Excursion permitted to 15°C - 30°C (59°F - 86°F). | |||
|packLabel=<!--Patient Counseling Information--> | |packLabel=<!--Patient Counseling Information--> | ||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
| Line 134: | Line 106: | ||
<!--Brand Names--> | <!--Brand Names--> | ||
|brandNames=* | |brandNames=* BENOQUIN®<ref>{{Cite web | title = BENOQUIN- monobenzone cream | url = http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=addb5a47-6693-4f77-8622-c2631c17a119}}</ref> | ||
<!--Look-Alike Drug Names--> | <!--Look-Alike Drug Names--> | ||
|drugShortage= | |drugShortage= | ||
}} | }} | ||
{{LabelImage | {{LabelImage | ||
|fileName= | |fileName=DailyMed - BENOQUIN- monobenzone cream .png | ||
}} | }} | ||
}} | }} | ||
<!--Pill Image--> | <!--Pill Image--> | ||
| Line 164: | Line 128: | ||
[[Category:Drug]] | [[Category:Drug]] | ||
[[Category:Citrates]] | |||
[[Category:Xanthines]] | |||
[[Category:Caffeine]] | |||
Latest revision as of 16:45, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Monobenzone is a hypopigmentation agent that is FDA approved for the treatment of for final depigmentation in extensive vitiligo. Common adverse reactions include achromia of skin, permanent, burning sensation, dermatitis, skin irritation, transient.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Benoquin Cream 20% is indicated for final depigmentation in extensive vitiligo.
- Benoquin Cream 20% is applied topically to permanently depigment normal skin surrounding vitiliginous lesions in patients with disseminated (greater than 50 percent of body surface area) idiopathic vitiligo.
- Benoquin Cream 20% is not recommended in freckling; hyperpigmentation caused by photosensitization following the use of certain perfumes (berlock dermatitis); melasma (chloasma) of pregnancy; or hyperpigmentation resulting from inflammation of the skin. Benoquin Cream 20% is not effective for the treatment of cafe-au-lait spots, pigmented nevi, malignant melanoma or pigmentation resulting from pigments other than melanin (e.g.: bile, silver, or artificial pigments).
- A thin layer of Benoquin Cream 20% should be applied and rubbed into the pigmented area two or three times daily, or as directed by physician. Prolonged exposure to sunlight should be avoided during treatment with Benoquin Cream 20%, or a sunscreen should be used.
- Depigmentation is usually accomplished after one to four months of Benoquin Cream 20% treatment. If satisfactory results are not obtained after four months of Benoquin Cream 20% treatment, the drug should be discontinued. When the desired degree of depigmentation is obtained, Benoquin Cream 20% should be applied only as often as needed to maintain depigmentation (usually only two times weekly).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Monobenzone in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Monobenzone in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Monobenzone in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Monobenzone in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Monobenzone in pediatric patients.
Contraindications
- Benoquin Cream 20% contains a potent depigmenting agent and is not a cosmetic skin bleach. Use of Benoquin Cream 20% is contraindicated in any conditions other than disseminated vitiligo. Benoquin Cream 20% frequently produces irreversible depigmentation, and it must not be used as a substitute for hydroquinone.
- Benoquin Cream 20% is also contraindicated in individuals with a history of sensitivity or allergic reactions to this product, or any of its ingredients.
Warnings
- Benoquin Cream 20% is a potent depigmenting agent, not a mild cosmetic bleach. Do not use except for final depigmentation in extensive vitiligo.
- Keep this, and all medications out of the reach of children. In case of accidental ingestion, call a physician or a Poison Control Center immediately.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Monobenzone in the drug label.
Postmarketing Experience
- Mild, transient skin irritation and sensitization, including erythematous and eczematous reactions have occurred following topical application of Benoquin Cream 20%. Although those reactions are usually transient, treatment with Benoquin Cream 20% should be discontinued if irritation, a burning sensation, or dermatitis occur. Areas of normal skin distant to the site of Benoquin Cream 20% application frequently have become depigmented, and irregular, excessive, unsightly, and frequently permanent depigmentation has occurred.
Drug Interactions
There is limited information regarding Monobenzone Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with Benoquin Cream 20%. It is also not known whether Benoquin Cream 20% can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. Benoquin Cream 20% should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Monobenzone in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Monobenzone during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Benoquin Cream 20% is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of Benoquin Cream 20% in pediatric patients below the age of 12 years have not been established.
Geriatic Use
There is no FDA guidance on the use of Monobenzone with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Monobenzone with respect to specific gender populations.
Race
There is no FDA guidance on the use of Monobenzone with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Monobenzone in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Monobenzone in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Monobenzone in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Monobenzone in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of Monobenzone in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Monobenzone in the drug label.
Overdosage
There is limited information regarding Monobenzone overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Monobenzone Pharmacology in the drug label.
Mechanism of Action
- Benoquin Cream 20% is a depigmenting agent whose mechanism of action is not fully understood.
- The topical application of monobenzone in animals, increases the excretion of melanin from the melanocytes. The same action is thought to be responsible for the depigmenting effect of the drug in humans. Monobenzone may cause destruction of melanocytes and permanent depigmentation. This effect is erratic and may take one to four months to occur while existing melanin is lost with normal sloughing of the stratum corneum. Hyperpigmented skin appears to fade more rapidly than does normal skin, and exposure to sunlight reduces the depigmenting effect of the drug. The histology of the skin after depigmentation with topical monobenzone is the same as that seen in vitiligo; the epidermis is normal except for the absence of identifiable melanocytes.
Structure
- Monobenzone is the monobenzyl ether of hydroquinone. Monobenzone occurs as a white, almost tasteless crystalline powder, soluble in alcohol and practically insoluble in water.
- Chemically, monobenzone is designated as p-(benzyloxy) phenol; the empirical formula is C13H12O2; molecular weight 200.24. The structural formula is:
- Each gram of Benoquin Cream contains 200 mg of monobenzone USP, in a water-washable base consisting of purified water USP, cetyl alcohol NF, propylene glycol USP, sodium lauryl sulfate NF and white wax NF.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Monobenzone in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Monobenzone in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Monobenzone in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Monobenzone in the drug label.
How Supplied
- Benoquin Cream 20% in 1 1/4 oz. tubes (35.4 g)
- (NDC 0187-0380-34).
Storage
- Benoquin Cream 20% should be stored at 25°C (77°F);
- Excursion permitted to 15°C - 30°C (59°F - 86°F).
Images
Drug Images
{{#ask: Page Name::Monobenzone |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Monobenzone |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Monobenzone in the drug label.
Precautions with Alcohol
- Alcohol-Monobenzone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- BENOQUIN®[1]
Look-Alike Drug Names
There is limited information regarding Monobenzone Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Monobenzone |Label Name=DailyMed - BENOQUIN- monobenzone cream .png
}}
}}