Mitral regurgitation pathophysiology: Difference between revisions
Rim Halaby (talk | contribs) |
Rim Halaby (talk | contribs) |
||
| Line 49: | Line 49: | ||
** [[Myxomatous degeneration]] (young patients) | ** [[Myxomatous degeneration]] (young patients) | ||
** Fibroelastic deficiency disease (old patients) | ** Fibroelastic deficiency disease (old patients) | ||
* Ischemic heart disease | |||
* [[Infective endocarditis]] | * [[Infective endocarditis]] | ||
* [[Connective tissue disease]] | * [[Connective tissue disease]] | ||
Revision as of 19:44, 3 September 2014
| Resident Survival Guide |
|
Mitral Regurgitation Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Mitral regurgitation pathophysiology On the Web |
|
American Roentgen Ray Society Images of Mitral regurgitation pathophysiology |
|
Risk calculators and risk factors for Mitral regurgitation pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Varun Kumar, M.B.B.S. ; Lakshmi Gopalakrishnan, M.B.B.S.; Mohammed A. Sbeih, M.D. [2].
Overview
Mitral regurgitation is due to either perforation or prolapse of the leaflets, dilation of the mitral annulus or rupture of the papillary muscles or chordae tendineae. There are several phases of mitral regurgitation (acute, chronic compensated, and chronic decompensated). In the acute phase, the volume and pressure overload in the left atrium is transmitted backward into the pulmonary vasculature to cause an elevation of the pulomanry capillary wedge pressure which causes dyspnea, PND, orthopnea and rales. During the chronic compensated phase of mitral regurgitation, the left ventricle maintains forward cardiac output by filling with a larger volume of blood than usual to accomodate the fact that a portion of the blood will go backwards into the left atrium. In the decompensated phase, the left ventricle begins to dilate and fail. The markers of decompensation are as follows: left ventricular end-diastolic dimension greater than 70 mm, left ventricular end-systolic dimension greater than 45 to 47 mm, and left ventricular ejection fraction (LVEF) less than 50 to 55 percent.
Anatomy
The mitral valve is composed of the valve leaflets, the mitral valve annulus (which forms a ring around the valve leaflets), the papillary muscles (which tether the valve leaflets to the left ventricle, preventing them from prolapsing into the left atrium), and the chordae tendineae (which connect the valve leaflets to the papillary muscles). A dysfunction of any of these portions of the mitral valve apparatus can cause mitral regurgitation.
The mitral annulus changes in shape and size during the cardiac cycle. It is smaller at the end of atrial systole due to the contraction of the left atrium around it, like a sphincter. This reduction in annulus size at the end of atrial systole may be important for the proper coapting of the leaflets of the mitral valve when the left ventricle contracts and pumps blood [1].
Physiology
Closure of the mitral valve and the tricuspid valve result in the first heart sound (S1). It is not actually the valve closure itself which produces the first heart sound but rather the sudden cessation of blood flow caused by the closure of the mitral and tricuspid valves. The mitral valve opening is normally not heard except in mitral stenosis (narrowing of the valve) as an opening snap. Flow of blood into the heart during rapid filling is not normally heard except in certain pathological states where it constitutes the third heart sound (S3).
Mechanical Basis of Mitral Regurgitation
The mechanical basis underlying mitral regurgitation includes the following:
- Anterior mitral leaflet prolapse
- Posterior mitral leaflet prolapse
- Bileaflet prolapse
- Restricted mitral leaflets
- Apical tethering
- Papillary muscle rupture
- Ischemic papillary muscle rupture
- Mitral leaflet perforation
- Rupture or tear of the chordae tendineae
- Dilation of the mitral annulus
- "Functional MR" due to dilation of the heart itself
Pathophysiology of Acute Mitral Regurgitation
Acute mitral regurgitation occurs when there is sudden disruption of one or more of the components of the mitral valve, such as leaflet perforation, rupture of a chordae tendineae, or rupture of the papillary muscle. The sudden disruption of the mitral valve may occur in the context of infective endocarditis, degenerative mitral valve disease, or acute ST elevation myocardial infarction. In the acute setting, the total stroke volume (i.e. the forward plus the regurgitant volume) is increased, but the forward cardiac output into the aorta is decreased because a proportion of the blood is going backward into the left atrium. Consequently, acute mitral regurgitation causes a sudden volume overload of both the left atrium and the left ventricle. As a result, pulmonary congestion and hypoxia occur following the elevation of the pressure in the left atrium.
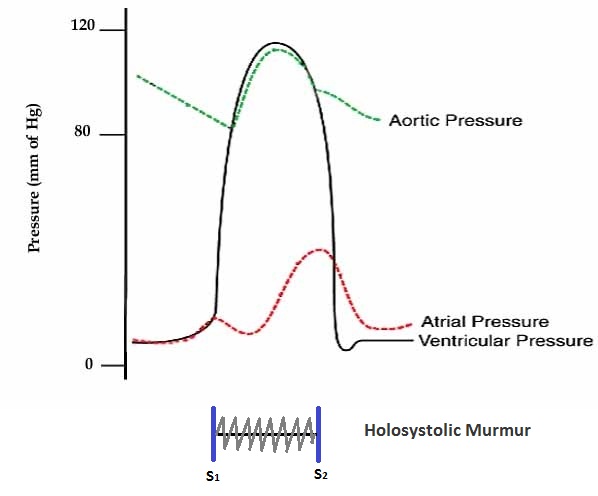
Shown below is an image depicting the pressures in the left atrium, left ventricle, and aorta in acute mitral regurgitation.
Pathophysiology of Chronic Mitral Regurgitation
Chronic MR can be classified into primary (degenerative) and secondary (functional) depending on the underlying pathophysiology.
Chronic primary MR results from chronic disruption of one or more component of the mitral valve (papillary muscles, chordae, and valve leaflets). This may occur among patients with:[2]
- Mitral valve prolapse
- Myxomatous degeneration (young patients)
- Fibroelastic deficiency disease (old patients)
- Ischemic heart disease
- Infective endocarditis
- Connective tissue disease
- Rheumatic heart disease
- Radiation heart disease
Chronic secondary MR results from left ventricular dysfunction and dilatation rather than an intrinsic abnormality in any of the components of the mitral valve. MR may be the result of an enlargement in the annular component of the mitral valve secondary to the dilatation of the left ventricle or the result of the displacement of the papillary muscle following remodelling of the left ventricle. Chronic secondary MR may occur in the setting of:[2]
- Coronary artery disease
- Any disease causing left ventricular dysfunction
- Idiopathic myocardial disease
Chronic Compensated Phase
- If the mitral regurgitation develops slowly over months to years or if the acute phase is successfully managed with medical therapy, the patient enters the chronic compensated phase of mitral regurgitation.
- In this phase, the left ventricle develops eccentric hypertrophy to reduce the wall stress associated with the rise in total stroke volume.
- The eccentric hypertrophy and the increased diastolic volume combine to increase the total stroke volume to levels well above normal, so that the forward stroke volume (forward cardiac output) is maintained.
- In the left atrium, the chronic volume overload causes left atrial enlargement, allowing the filling pressure in the left atrium to decrease. This dilation of the left atrium reduces the pulmonary capillary wedge pressure, and is associated with an improvement in the signs (e.e. rales) and symptoms (dyspnea, PND, and orthopnea) of pulmonary congestion.
- These compensatory changes in the left ventricle and left atrium maintain the forward cardiac output of the left ventricle, and minimize the signs and symptoms of pulmonary congestion that occur in the acute phase of the disease. Individuals in the chronic compensated phase may be asymptomatic and have normal exercise tolerance.
- In the compensated stage; the left ventricular (LV) end-diastolic dimension is less than 60 mm, and the end-systolic dimension is less than 40 mm on echocardiography.
Chronic Decompensated Phase
- An individual may remain in the compensated phase of mitral regurgitation for years, but will eventually develop left ventricular dysfunction, the hallmark for the chronic decompensated phase of mitral regurgitation. It is currently unclear what causes an individual to enter the decompensated phase of this disease. However, the decompensated phase is characterized by calcium overload within the cardiac myocytes.
- In this phase, the ventricular myocardium is no longer able to contract adequately to compensate for the volume overload of mitral regurgitation, and the stroke volume of the left ventricle begins to decrease.
- The reduced stroke volume causes a decrease in the forward cardiac output and an increase in the end-systolic volume.
- The increased left ventricular end-systolic volume in turn causes increased left ventricular end diastolic pressures and increased pulmonary capillary wedge pressures.
- As the wedge pressure rises, the patient may develop symptoms of congestive heart failure such as dyspnea, PND and orthopnea and signs of congestive heart failure including rales.
- With the rise in wall stress that accompanies the rise in pressure and volume in the left ventricle, the left ventricle begins to dilate during this phase. This causes a dilatation of the mitral valve annulus, which may further worsen the degree of mitral regurgitation.
- While the ejection fraction is less in the chronic decompensated phase than in the acute phase or the chronic compensated phase of mitral regurgitation, it may still be in the normal range (i.e: > 50 percent), and may not decrease until late in the disease course. A decreased ejection fraction in an individual with mitral regurgitation and no other cardiac abnormality should alert the physician that the disease may be in its decompensated phase.
- The decompensated stage defined on the basis of decompensated ventricular function. At this stage; the patients are at risk for a poor results of valve replacement.
It is helpful to classify the stage of the patient's disease so as to recognize signs that may indicate that the patient is transitioning into the decompensated phase of the disease. The goal is to perform mitral valve surgery before the patient transitions into the decompensated phase. Once the patient transitions into the decompensated phase, (left ventricular enlargement and a low left ventricular ejection fraction) there may not be recovery of left ventricular funtion following operative repair or replacement of the mitral valve.
Markers of Decompensated Ventricular Function in Mitral Regurgitaiton
- Left ventricular end-diastolic dimension greater than 70 mm
- Left ventricular end-systolic dimension greater than 45 to 47 mm
- Left ventricular ejection fraction (LVEF) less than 50 to 55 percent
Summary Chart Distinguishing Acute and Chronic Mitral Regurgitation
| Acute mitral regurgitation | Chronic mitral regurgitation | |
|---|---|---|
| Electrocardiogram | Normal | P mitrale, atrial fibrillation, left ventricular hypertrophy |
| Heart size | Normal | Cardiomegaly, left atrial enlargement |
| Systolic murmur | Heard at the base, radiates to the neck, spine, or top of head | Heard at the apex, radiates to the axilla |
| Apical thrill | May be absent | Present |
| Jugular venous distension | Present | Absent |
References
- ↑ Pai RG, Varadarajan P, Tanimoto M (2003). "Effect of atrial fibrillation on the dynamics of mitral annular area". J Heart Valve Dis. 12 (1): 31–7. PMID 12578332.
- ↑ 2.0 2.1 Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA; et al. (2014). "2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines". J Am Coll Cardiol. 63 (22): 2438–88. doi:10.1016/j.jacc.2014.02.537. PMID 24603192.