Methylene blue: Difference between revisions
No edit summary |
No edit summary |
||
| Line 62: | Line 62: | ||
| ChEBI_Ref = {{ebicite|correct|EBI}} | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| ChEBI = 6872 | | ChEBI = 6872 | ||
| SMILES = CN(C)c1ccc2c(c1)sc-3cc(= | | SMILES = CN(C)c1ccc2c(c1)sc-3cc(=N+(C)C)ccc3n2.Cl- | ||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| ChEMBL = 405110 | | ChEMBL = 405110 | ||
Revision as of 13:29, 23 April 2015
{{DrugProjectFormSinglePage |authorTag=Turky Alkathery, M.D. [1] |genericName=Methylene blue |indicationType=treatment |indication=Drug induced methemoglobinemia |adverseReactions=Hypertension, hypotension, sweating symptom, abdominal pain, diarrhea, nausea, vomiting, dizziness, headache and confusion |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content) |fdaLIADAdult====Indications===
- Drug induced methemoglobinemia
Dosage
- 0.1 to 0.2 mL per kilogram of body weight (0.045 to 0.09 mL per pound of body weight). Inject Methylene Blue intravenously very slowly over a period of several minutes.
|offLabelAdultGuideSupport=*There is limited information regarding Off-Label Guideline-Supported Use of Methylene blue in adult patients. |offLabelAdultNoGuideSupport=*There is limited information regarding Off-Label Non–Guideline-Supported Use of Methylene blue in adult patients. |fdaLIADPed=Safety and effectiveness in pediatric patients have not been established. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Methylene blue in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Methylene blue in pediatric patients. |contraindications=*Methylene blue can cause fetal harm when administered to a pregnant woman. An association exists between the use of methylene blue in amniocentesis and atresia of the ileum and jejunum, ileal occlusions and other adverse effects in the neonate. Methylene blue is contraindicated in women who are or may become pregnant. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus.
- Intraspinal injection is contraindicated.
- Methylene blue is contraindicated in patients with a known hypersensitivity to the drug.
|warnings=*Methylene Blue should not be given by subcutaneous or intrathecal injection.
- Methylene blue is a potent monoamine oxidase inhibitor: Methylene blue has been demonstrated to be a potent monoamine oxidase inhibitor (MAOI) and may cause potentially fatal serotonin toxicity (serotonin syndrome) when combined with serotonin reputake inhibitors (SRIs). (4) Serotonin toxicity is characterized by development of neuromuscular hyperactivity (tremor, clonus, myoclonus and hyperreflexia, and, in the advanced stage, pyramidal rigidity); autonomic hyperactivity (diaphoresis, fever, tachycardia, tachypnoea, and mydraisis); and altered mental status (agitation, excitement, and in the advanced stage, confusion). If methylene blue is judged to be indicated, SRIs must be ceased, prior to treatment/procedure/surgery.
Precautions
- Glucose-6-Phosphate Dehydrogenase Deficiency (G6PD): Methylene blue should be avoided in patients with G6PD deficiency due to the risk of paradoxical methemoglobinemia and hemolysis.
|clinicalTrials=*Large intravenous doses of Methylene Blue produce nausea, abdominal and precordial pain, dizziness, headache, profuse sweating, mental confusion and the formation of methemoglobin. |postmarketing=*There is limited information regarding postmarketing experience |drugInteractions=*Methylene blue may interact with any drug that acts as a serotonin reuptake inhibitor (SRI) including, amongst others, selective serotonin reuptake inhbitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), norepinephrine-dopamine reuptake inhibitors (NDRIs), triptans and ergot alkaloids; such combinations may have the consequence of potentially fatal serotonin toxicity (serotonin syndrome). Methylene blue should not be co-administered with any drug that acts as an SRI. |FDAPregCat=X |useInPregnancyFDA=*Epidemiologic evidence exists that Methylene blue is a teratogen. An association exists between the use of methylene blue in amniocentesis and atresia of the ileum and jejunum, ileal occlusions and other adverse effects in the neonate. Methylene blue Injection should not be administered to pregnant women during amniocentesis due to the risk of teratogenicity and other newborn adverse effects |useInRenalImpair=*Renal Failure: Methylene blue should be used with caution in patients with severe renal impairment . |administration=*Intravenous. |monitoring=*There is limited information regarding drug monitoring. |IVCompat=*There is limited information regarding IV compatibility. |drugBox=
Template:Chembox E number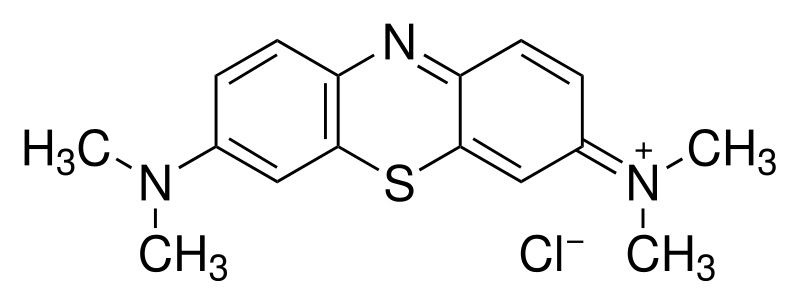
| |
| |
| Names | |
|---|---|
| IUPAC names
3,7-bis(Dimethylamino)-
phenothiazin-5-ium chloride | |
| Other names
C.I. 52015
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| UNII | |
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |