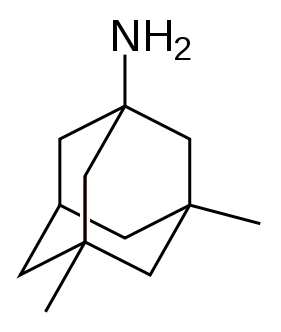
Memantine
 | |
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100% |
| Metabolism | Hepatic (<10%) |
| Elimination half-life | 60–100 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C12H21N |
| Molar mass | 179.3 g/mol |
|
WikiDoc Resources for Memantine |
|
Articles |
|---|
|
Most recent articles on Memantine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Memantine at Clinical Trials.gov Clinical Trials on Memantine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Memantine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Memantine Discussion groups on Memantine Directions to Hospitals Treating Memantine Risk calculators and risk factors for Memantine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Memantine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Memantine is the first in a novel class of Alzheimer's disease medications acting on the glutamatergic system. Memantine was developed by Merz and licensed to Forest for the U.S. and Lundbeck for selected European and international markets. Memantine is marketed under the brands Axura® and Akatinol® by Merz, Namenda® by Forest and Ebixa® by Lundbeck.
Pharmacology
Glutamatergic (NMDA receptor)
A dysfunction of glutamatergic neurotransmission, manifested as neuronal excitotoxicity, is hypothesized to be involved in the etiology of Alzheimer's disease. Targeting the glutamatergic system, specifically NMDA receptors, offers a novel approach to treatment in view of the limited efficacy of existing drugs targeting the cholinergic system.[1]
Memantine is a low-affinity voltage-dependent uncompetitive antagonist at glutamatergic NMDA receptors. [2] [3] By binding to the NMDA receptor with a higher affinity than Mg2+ ions, memantine is able to inhibit the prolonged influx of Ca2+ ions which forms the basis of neuronal excitotoxicity. The low affinity and rapid kinetics of memantine at the level of the NMDA receptor-channel, however, preserves the physiological function of the receptor as it can still be activated by the relatively high concentrations of glutamate released following depolarization of the presynaptic neuron. [4] Whether the interaction of memantine with NMDA receptors plays a role in the symptomatic improvement the drug produces in Alzheimer's disease is a matter of speculation. Moreover, there is no evidence as yet that the ability of memantine to protect against NMDA receptor-mediated excitotoxicity has a disease modifying effect in Alzheimer's.
Serotonergic (5-HT3 receptor)
Memantine acts as an uncompetitive antagonist at the 5HT3 receptor, with a potency similar to that for the NMDA receptor.[5] The clinical significance of this serotonergic activity in the treatment of Alzheimer's disease is unknown.
Cholinergic (Nicotinic acetylcholine receptor)
Memantine acts as an uncompetetive antagonist at different neuronal nicotinic neuronal receptors (nAChRs) at potencies similar to the NMDA receptor.[6][7] It has been shown that the number of nicotinic receptors in the brain are reduced in Alzheimer's disease, even in the absence of a general decrease in the number of neurons, and nicotinic receptor agonists are viewed as interesting targets for anti-Alzheimer drugs.[8]
Clinical use
Indications
Although memantine is approved for treatment of moderate to severe Alzheimer's Disease[9] its usage has been recommended against by the UK's National Institute for Clinical Excellence.[10]
Memantine has been associated with a moderate decrease in clinical deterioration in Alzheimer's disease.[11] A systematic review of randomised controlled trials found that memantine has a small positive effect on cognition, mood, behaviour, and the ability to perform daily activities in moderate to severe Alzheimer's disease, but an unknown effect in mild to moderate disease.[12]
Memantine is also being tested for Opioid dependence, systemic lupus erythematosus, depression, obsessive compulsive disorder, glaucoma, tinnitus, neuropathic pain, and pervasive developmental disorders.
Adverse drug reactions
Memantine is generally well-tolerated.[12] Common adverse drug reactions (≥1% of patients) include: confusion, dizziness, drowsiness, headache, insomnia, agitation, and/or hallucinations. Less common adverse effects include: vomiting, anxiety, hypertonia, cystitis, and increased libido.[13][11]
See also
References
- ↑ Cacabelos R, Takeda M, Winblad B. The glutamatergic system and neurodegeneration in dementia: preventive strategies in Alzheimer's disease puku. Int J Geriatr Psychiatry 1999;14(1):3-47. PMID 10029935
- ↑ Rogawski, MA (2003). "The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease". CNS Drug Rev. 9 (3): 275–308. PMID 14530799. Unknown parameter
|coauthors=ignored (help) - ↑ Robinson, DM (2006). "Memantine: a review of its use in Alzheimer's disease". Drugs. 66 (11): 1515–1534. PMID 16906789. Unknown parameter
|coauthors=ignored (help);|access-date=requires|url=(help) - ↑ Rogawski, MA (2000). "Low affinity channel blocking (uncompetitive) NMDA receptor antagonists as therapeutic agents--toward an understanding of their favorable tolerability". Amino Acids. 19 (1): 133–149. PMID 11026482.
- ↑ Rammes G, Rupprecht R, Ferrari U, Zieglgansberger W, Parsons CG. The N-methyl-D-aspartate receptor channel blockers memantine, MRZ 2/579 and other amino-alkyl-cyclohexanes antagonise 5-HT(3) receptor currents in cultured HEK-293 and N1E-115 cell systems in a non-competitive manner. Neurosci Lett 2001;306(1-2):81-4. PMID 11403963
- ↑ Buisson B, Bertrand D. Open-channel blockers at the human α4β2 neuronal nicotinic acetylcholine receptor. Mol Pharmacol 1998;53:555–63. PMID 9495824
- ↑ Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks α7* nicotinic acetylcholine receptors more potently than N-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther 2005;312:1195–205. PMID 15522999
- ↑ Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol 2004;74:363–96. PMID 15649582
- ↑ Mount C, Downton C. Alzheimer's Disease: Progress or Profit?. Nature Medicine 2006 (12) 3: 1280-1284. PMID 16829947
- ↑ NICE technology appraisal guidance 111 Donepezil, galantamine, rivastigmine (review) and memantine for the treatment of Alzheimer’s disease
- ↑ 11.0 11.1 Rossi S, editor. Australian Medicines Handbook 2006. Adelaide: Australian Medicines Handbook; 2006.
- ↑ 12.0 12.1 Areosa SA, Sherriff F, McShane R. Memantine for dementia. Cochrane Database Syst Rev 2005;(3):CD003154. PMID 16034889
- ↑ Joint Formulary Committee. British National Formulary. 47th ed. London: British Medical Association and the Royal Pharmaceutical Society of Great Britain; 2004. ISBN 0-85369-584-9
Template:Anti-dementia drugs Template:Dissociative hallucinogens
- Pages with script errors
- Pages with citations using unsupported parameters
- Pages using citations with accessdate and no URL
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Antidementia agents
- NMDA receptor antagonists
- Drugs
- Psychiatry
- Neurology