Maprotiline: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{PB}} | |authorTag={{PB}} | ||
|genericName=Maprotiline | |||
|aOrAn=a | |aOrAn=a | ||
|drugClass=tetracyclic antidepressant | |||
|indication=bipolar disorder, depressed phase, depression, dysthymia, mixed anxiety and depressive disorder | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | ||
|fdaLIADAdult= | |fdaLIADAdult=* Bipolar disorder, depressed phase | ||
:*Outpatients: 75 mg/day PO (2-3 divided doses) for 2 weeks; may increase in 25 mg increments up to a max of 225 mg/day in single or divided doses | |||
* | :*Inpatients: 100-150 mg/day PO (2-3 divided doses) for 2 weeks; may increase in 25 mg increments up to a max of 225 mg/day in single or divided doses | ||
:*Maintenance: 75-150 mg/day PO in single or divided doses | |||
:: ( | *Depression | ||
:* | |||
*Dysthymia | |||
* | :* | ||
*Mixed anxiety and depressive disorder | |||
* | :* | ||
|offLabelAdultNoGuideSupport=*Pain | |||
* | |||
* | |||
:* | |||
|offLabelAdultNoGuideSupport= | |||
* | |||
:* There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Maprotiline in adult patients. | :* There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Maprotiline in adult patients. | ||
|offLabelPedNoGuideSupport=:* There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Maprotiline in pediatric patients. | |||
|offLabelPedNoGuideSupport= | |||
:* There is limited information about <i>Off-Label Non–Guideline-Supported Use</i> of Maprotiline in pediatric patients. | |||
|contraindications=* Condition 1 | |contraindications=* Condition 1 | ||
* Condition 2 | * Condition 2 | ||
| Line 108: | Line 83: | ||
* (Drug 3) | * (Drug 3) | ||
:* (Description) | :* (Description) | ||
|drugBox={{drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 477169275 | |||
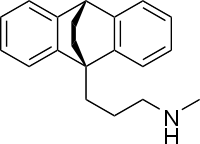
| IUPAC_name = ''N''-Methyl-9,10-ethanoanthracene-9(10''H'')-propanamine | |||
| image = 200px-Maprotiline.svg.png | |||
| width = 200 | |||
| image2 = 250px-Maprotiline3Dan.gif | |||
| width2 = 250 | |||
<!--Clinical data--> | |||
| Drugs.com = {{drugs.com|monograph|maprotiline-hydrochloride}} | |||
| MedlinePlus = a682158 | |||
| pregnancy_US = B3 | |||
| legal_AU = S4 | |||
| legal_US = Rx-only | |||
| routes_of_administration = oral, intramuscular, intravenous (infusion) | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 66 to 70% | |||
| protein_bound = 88% | |||
| metabolism = hepatic | |||
| elimination_half-life = 27-58 hours | |||
| excretion = biliar (30%) and urine (57%) as gluconurides, 3 to 4% as unchanged drug | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 10262-69-8 | |||
| ATC_prefix = N06 | |||
| ATC_suffix = AA21 | |||
| ATC_supplemental = | |||
| PubChem = 4011 | |||
| IUPHAR_ligand = 2402 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00934 | |||
| ChemSpiderID = 23719117 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 2U1W68TROF | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02566 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 21731 | |||
<!--Chemical data--> | |||
| C=20 | H=23 | N=1 | |||
| molecular_weight = 277.403 g/mol | |||
| smiles = CNCCC[C@@]12C3=CC=CC=C3[C@@H](C4=CC=CC=C14)CC2 | |||
| InChI = 1/C20H23N/c1-21-14-6-12-20-13-11-15(16-7-2-4-9-18(16)20)17-8-3-5-10-19(17)20/h2-5,7-10,15,21H,6,11-14H2,1H3/t15-,20+ | |||
| InChIKey = QSLMDECMDJKHMQ-GSXCWMCIBU | |||
| StdInChI = 1S/C20H23N/c1-21-14-6-12-20-13-11-15(16-7-2-4-9-18(16)20)17-8-3-5-10-19(17)20/h2-5,7-10,15,21H,6,11-14H2,1H3/t15-,20+ | |||
| StdInChIKey = QSLMDECMDJKHMQ-GSXCWMCISA-N | |||
}} | |||
|mechAction=It exerts blocking effects at the following postsynaptic receptors: | |mechAction=It exerts blocking effects at the following postsynaptic receptors: | ||
* Strong : alpha<sub>1</sub> | * Strong : alpha<sub>1</sub> | ||
Revision as of 19:10, 26 May 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Maprotiline is a tetracyclic antidepressant that is FDA approved for the {{{indicationType}}} of bipolar disorder, depressed phase, depression, dysthymia, mixed anxiety and depressive disorder. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Bipolar disorder, depressed phase
- Outpatients: 75 mg/day PO (2-3 divided doses) for 2 weeks; may increase in 25 mg increments up to a max of 225 mg/day in single or divided doses
- Inpatients: 100-150 mg/day PO (2-3 divided doses) for 2 weeks; may increase in 25 mg increments up to a max of 225 mg/day in single or divided doses
- Maintenance: 75-150 mg/day PO in single or divided doses
- Depression
- Dysthymia
- Mixed anxiety and depressive disorder
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
- Pain
- There is limited information about Off-Label Non–Guideline-Supported Use of Maprotiline in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Maprotiline FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Non–Guideline-Supported Use
- There is limited information about Off-Label Non–Guideline-Supported Use of Maprotiline in pediatric patients.
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Drug Interactions
- (Drug 1)
- (Description)
- (Drug 2)
- (Description)
- (Drug 3)
- (Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Maprotiline in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Maprotiline in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Maprotiline during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Maprotiline in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Maprotiline in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Maprotiline in geriatric settings.
Gender
There is no FDA guidance on the use of Maprotiline with respect to specific gender populations.
Race
There is no FDA guidance on the use of Maprotiline with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Maprotiline in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Maprotiline in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Maprotiline in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Maprotiline in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Maprotiline Administration in the drug label.
Monitoring
There is limited information regarding Maprotiline Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Maprotiline and IV administrations.
Overdosage
There is limited information regarding Maprotiline overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
It exerts blocking effects at the following postsynaptic receptors:
- Strong : alpha1
- Moderate : 5-HT2, muscarinic, H1, D2
- Weak : alpha2
- Extremely weak : 5-HT1
The pharmacologic profile of Maprotiline explains its antidepressant, sedative, anxiolytic, sympatholytic, and anticholinergic activities. Additionally, it shows a strong antagonism against Reserpine-induced effects in animal studies, as do the other 'classical' antidepressants. Although Maprotiline behaves in most regards as a 'first generation antidepressant' it is commonly referred to as 'second generation antidepressant'.
Sedation has a fast onset (the same day), while remission of the depression itself is noted usually after a latent period of one to four weeks.
Maprotiline does not brighten up the mood in nondepressed persons.
Structure
There is limited information regarding Maprotiline Structure in the drug label.
Pharmacodynamics
There is limited information regarding Maprotiline Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Maprotiline Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Maprotiline Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Maprotiline Clinical Studies in the drug label.
How Supplied
There is limited information regarding Maprotiline How Supplied in the drug label.
Storage
There is limited information regarding Maprotiline Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Maprotiline |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Maprotiline |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
For patient information, please click here.
Precautions with Alcohol
Alcohol-Maprotiline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Maprotiline Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Maprotiline Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.