Malachite green
|
WikiDoc Resources for Malachite green |
|
Articles |
|---|
|
Most recent articles on Malachite green Most cited articles on Malachite green |
|
Media |
|
Powerpoint slides on Malachite green |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Malachite green at Clinical Trials.gov Trial results on Malachite green Clinical Trials on Malachite green at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Malachite green NICE Guidance on Malachite green
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Malachite green Discussion groups on Malachite green Patient Handouts on Malachite green Directions to Hospitals Treating Malachite green Risk calculators and risk factors for Malachite green
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Malachite green |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Malachite green, also called aniline green, basic green 4, diamond green B, or victoria green B, IUPAC name:4-[(4-dimethylaminophenyl)-phenyl-methyl]-N,N-dimethyl-aniline is a toxic chemical primarily used as a dye. When diluted, it can be used as a topical antiseptic or to treat parasites, fungal infections, and bacterial infections in fish and fish eggs. It is also used as a bacteriological stain.
However, in 1992 in Canada, it was determined that there is a significant health risk to humans who eat fish contaminated with malachite green. The chemical was classified a Class II Health Hazard because it was found to be toxic to human cells and might cause liver tumor formation. However, due to its ease and low cost to manufacture, it is still used in certain countries with less restrictive laws for non-aquaculture purposes. In 2005 eels and fish imported from China and Taiwan were found in Hong Kong with traces of this chemical. Also, in 2006 the United States Food and Drug Administration (FDA) detected malachite green in seafood imported into that country for human consumption by China, where the substance is also banned for use in aquaculture. In June 2007, the FDA blocked the importation of several varieties of seafood due to continued malachite green contamination.[3][4] The substance has been banned in the United States since 1983 in food-related applications.
Source: http://chemistry.tidalswan.com
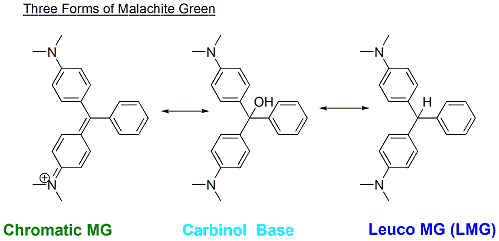
The structures of compounds explain their chemical and biological properties, such as how well they might be absorbed in the body and how reactive they are. Malachite green is commonly known in a form called the chromatic form in which it is a green dye. However, as it is absorbed into the body, it is converted by body mechanisms into other forms that are equally important for us to consider. The first form is called the carbinol form, which is important because it spreads across cell membranes faster. When it is inside the cell, it is then metabolized into a form called leuco-malachite green. This form is known by researchers to be toxic in addition to the fact that it is retained in the body for a longer period than the chromatic form of malachite green.
Chromatic form of malachite green
Template:PH indicator template
Template:PH indicator template Malachite green is used to dye materials like silk, leather, and paper. The chemical known as malachite green does not actually contain the mineral malachite — the name comes from the similarity of color.
Malachite green is also found to be especially active against the fungus Saprolegnia, which infects fish eggs in commercial aquaculture. It is also a very popular treatment against ichthyophthirius in freshwater aquaria. The principle metabolite, leuco-malachite green (LMG), is the main chemical found in fish treated with malachite green. This is due to its longer retention time inside fish muscle tissues.
The use of this substance has been banned in many countries as a suspected carcinogen. Lab tests revealed that rats fed malachite green at the concentration of 100 ppb for longer than 2 years showed signs of tumors.
Malachite green is known to be highly toxic to certain freshwater fish such as tetras, catfish and shark catfish. It is strongly recommended that half-dosage be observed in treating freshwater tanks with catfish, tetras, scaleless, and other bottom feeder fish.
Malachite green is used as a biological stain for microscopic analysis of cell and tissue samples. In the Gimenez staining method, basic fuchsin stains bacteria red or magenta, and malachite green is used as a blue-green counterstain. Malachite green can also directly stain endospores within cells; here a safranin counterstain is often used.
Malachite green can also be used as a saturable absorber in dye lasers, or as a pH indicator between pH 0.2 - 1.8. However this use is relatively rare.
Leuco-malachite green (LMG) is used as a detection method for latent blood in criminalistics. Hemoglobin catalyzes the reaction between LMG and hydrogen peroxide, converting the colorless LMG to the chromatic form of malachite green. Therefore, the appearance of a green color indicates the presence of blood.
Toxicity of malachite green
When malachite green is used in aquatic animals, it will be metabolized to leuco-malachite green. The non-polar LMG has been found to retain in catfish muscle for a longer period of them, 10 days for LMG compared to 2.8 in MG. It has been determined that the half lives of the retention of malachite green and leuco-malachite green catfish muscle is 2.8 days.1
The study of the toxicity of malachite green in fish has been hard as it is heavily influenced by the water hardness, pH, temperature and amount of dissolved oxygen in water. Detailed studies have indicated that the toxicity of the chemical increases as the temperature increases or pH decreases. The effects of malachite green on fish eggs have also been tested and it has been shown that a twofold increase in the concentration of malachite green could lead up to 20 times the mortality rate in rainbow trout eggs. This shows that it may be extremely toxic for some species of fish and especially for fish eggs. Other effects such as carcinogenesis, mutagenesis, and reduced fertility have been reported to occur in rainbow trout. Overall, although malachite green is an extremely effective weapon against fungus and parasitic infections in fish, the chemical causes serious side effects in the fish as well.
Effects on humans
Malachite green and its major metabolite, leuco-malachite green has been reported to have mutagenic and carcinogenic effects. Culp SJ in her recent article published in Mutation Research mentions that rats fed malachite green experience “a dose-related increase in liver DNA adducts” along with lung adenomas. Leuco-malachite green causes an “increase in the number and severity of changes”. As leuco-malachite green is the primary metabolite of malachite green and is retained in fish muscle much longer, most intake of malachite green would be in the leuco form. During the experiment, rats were fed up to 543 ppm of leuco-malachite green, an extreme amount compared to the average 5 ppb discovered in fish. After a period of two years, an increase in lung adenomas in male rats was discovered but no incidences of liver tumors. This shows that although adducts are formed, they have “little mutagenic or carcinogenic consequence.” Therefore it could be concluded that malachite green caused carcinogenic symptoms but a direct link between malachite green and liver tumor could not be proved.
Method of mutagenic activity
Taken together, these data suggested that the N-demethylated metabolites of leuco-malachite green and malachite green could undergo metabolic activation in a manner similar to that observed with carcinogenic aromatic amines, i.e. oxidation to metabolites that react with DNA either directly or after esterification. However, the adduct has not been characterized, since existing in vitro mutagenicity assays and metabolic activation systems has been unsuccessful in activating leuco-malachite green or malachite green to DNA-damaging species (Culp and Blankenship, unpublished results).
Cases involving malachite green detected in fish
An investigation by the Hong Kong Government Labs (Hong Kong Health Department) in 2005 revealed freshwater fish, crabs and other aquaculture products in China had small traces of malachite green. Later, saltwater fish from China and Taiwan were also found to contain this toxin. However, Taiwan officials asserted this discovery to be unconfirmable, stating that malachite green has long been banned in Taiwan.[5][6] Hong Kong’s Food & Environmental Hygiene Department confirmed that 11 of 14 eel-based products tested from local supermarkets had high levels of malachite green. However, the concentration of malachite green found in seafood was extremely small, with the highest concentration in eels found to be 4,500 μg/kg and 900 μg/kg for freshwater fish.
Sources
- Bongsup P. Cho et al, Chem. Res. Toxicol., 16 (3), 285 -294, (2003). 10.1021 - Found important information about synthesis of molecule.
- S.M. Plakas, et al, Can. J. Fish. Aquat. Sci. 53: 1427.1433 (1996). - Found general background information about toxicity of malachite green and its uptake in Catfish. Information was extremely specific but effects of pH on malachite green was used in the "Detection" area of site.
- S.J. Culp et al. / Mutation Research 506–507 (2002) 55–63 - Detailed information about toxicity of malachite green.
- S. M. Plakas, K. R. El Said, G. R. Stehly, W. H. Gingerich and J. H. Allen, Can. J. Fish. Aquat. Sci. 53, 1427–1433 (1996).
- Schoettger, 1970; Smith and Heath, 1979; Gluth and Hanke, 1983. Bills et al. (1977)
- Research done on eggs and fry of large mouth bass, Micropterus salmonides.
External links
- Site about malachite green
- U.S. National Institutes of Health
- University of Guelph
- U.S. Food and Drug Administration
- U.K. Department of Health
- Wiki Article on Malachite Green
de:Malachitgrün ko:말라카이트 그린 sv:Malakitgrönt (veterinärmedicin)