Lisdexamfetamine: Difference between revisions
(Blanked the page) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{SS}} | |||
|genericName=Lisdexamfetamine | |||
|aOrAn=an | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Lisdexamfetamine in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Lisdexamfetamine in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Lisdexamfetamine in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Lisdexamfetamine in pediatric patients. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=<u>Risk Summary</u> | |||
There are no adequate and well-controlled studies with Vyvanse in pregnant women. Adverse pregnancy outcomes, including premature delivery and low birth weight, have been seen in infants born to mothers dependent on amphetamines. Long-term neurochemical and behavioral effects have been reported in animal developmental studies using clinically relevant doses of amphetamine (d- or d,l-). Animal reproduction studies performed with lisdexamfetamine dimesylate in rats and rabbits showed no effects on embryofetal morphological development and survival. Vyvanse should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
<u>Clinical Considerations</u> | |||
Amphetamines, such as Vyvanse, cause vasoconstriction and thereby may decrease placental perfusion. Infants born to amphetamine-dependent mothers have an increased risk of premature delivery and low birth weight. | |||
Monitor infants born to mothers taking amphetamines for symptoms of withdrawal such as feeding difficulties, irritability, agitation, and excessive drowsiness. | |||
<u>Human Data</u> | |||
Available data in women using amphetamines during pregnancy do not show a clear increased risk of major congenital malformations. Two case control studies of over a thousand patients in total exposed to amphetamines at different gestational ages did not show an increase in congenital abnormalities. | |||
<u>Animal Data</u> | |||
Lisdexamfetamine dimesylate had no apparent effects on embryofetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis at doses of up to 40 and 120 mg/kg/day, respectively. These doses are approximately 4 and 27 times, respectively, the maximum recommended human dose of 70 mg/day given to adolescents, on a mg/m2 body surface area basis. | |||
A number of studies in rodents indicate that prenatal or early postnatal exposure to amphetamine (d- or d,l-) at doses similar to those used clinically can result in long-term neurochemical and behavioral alterations. Reported behavioral effects include learning and memory deficits, altered locomotor activity, and changes in sexual function. | |||
|useInNursing=Amphetamines are excreted into human milk. Long-term neurodevelopmental effects on infants from amphetamine exposure are unknown. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=Safety and effectiveness have been established in pediatric patients with ADHD ages 6 to 17 years [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. Safety and efficacy in pediatric patients below the age of 6 years have not been established. | |||
<u>Growth Suppression</u> | |||
Growth should be monitored during treatment with stimulants, including Vyvanse, and children who are not growing or gaining weight as expected may need to have their treatment interrupted | |||
<u>Juvenile Animal Data</u> | |||
Studies conducted in juvenile rats and dogs at clinically relevant doses showed growth suppression that partially or fully reversed in dogs and female rats but not in male rats after a four-week drug-free recovery period. | |||
A study was conducted in which juvenile rats received oral doses of 4, 10, or 40 mg/kg/day of lisdexamfetamine dimesylate from day 7 to day 63 of age. These doses are approximately 0.3, 0.7, and 3 times the maximum recommended human daily dose of 70 mg on a mg/m2 basis for a child. Dose-related decreases in food consumption, bodyweight gain, and crown-rump length were seen; after a four-week drug-free recovery period, bodyweights and crown-rump lengths had significantly recovered in females but were still substantially reduced in males. Time to vaginal opening was delayed in females at the highest dose, but there were no drug effects on fertility when the animals were mated beginning on day 85 of age. | |||
In a study in which juvenile dogs received lisdexamfetamine dimesylate for 6 months beginning at 10 weeks of age, decreased bodyweight gain was seen at all doses tested (2, 5, and 12 mg/kg/day, which are approximately 0.5, 1, and 3 times the maximum recommended human daily dose on a mg/m2 basis for a child). This effect partially or fully reversed during a four-week drug-free recovery period. | |||
|useInGeri=Clinical studies of Vyvanse did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
|othersTitle=Special Populations | |||
|useInOthers=[[File:lisdexamfetamine_Special Populations_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|administration=<h4>General Instructions for Use</h4> | |||
Take Vyvanse by mouth in the morning with or without food; avoid afternoon doses because of the potential for insomnia. Vyvanse may be administered in one of the following ways: | |||
* Swallow Vyvanse capsules whole, or | |||
* Open capsules, empty and mix the entire contents with yogurt, water, or orange juice. If the contents of the capsule include any compacted powder, a spoon may be used to break apart the powder. The contents should be mixed until completely dispersed. Consume the entire mixture immediately. It should not be stored. The active ingredient dissolves completely once dispersed; however, a film containing the inactive ingredients may remain in the glass or container once the mixture is consumed. Do not take anything less than one capsule per day, and a single capsule should not be divided. | |||
|monitoring=FDA Package Insert for Lisdexamfetamine contains no information regarding drug monitoring. | |||
|IVCompat=There is limited information about the IV Compatibility. | |||
|overdose=Consult with a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice for treatment of overdosage. Individual patient response to amphetamines varies widely. Toxic symptoms may occur idiosyncratically at low doses. | |||
Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Other reactions include arrhythmias, hypertension or hypotension, circulatory collapse, nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma. | |||
==DRUG ABUSE AND DEPENDENCE== | |||
===Controlled Substance=== | |||
Vyvanse contains lisdexamfetamine, a prodrug of amphetamine, a Schedule II controlled substance. | |||
===Abuse=== | |||
CNS stimulants, including Vyvanse, other amphetamines, and methylphenidate-containing products have a high potential for abuse. Abuse is characterized by impaired control over drug use, compulsive use, continued use despite harm, and craving. | |||
Signs and symptoms of CNS stimulant abuse may include increased heart rate, respiratory rate, blood pressure, and/or sweating, dilated pupils, hyperactivity, restlessness, insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and/or abdominal pain. Anxiety, psychosis, hostility, aggression, suicidal or homicidal ideation have also been seen. Abusers of CNS stimulants may chew, snort, inject, or use other unapproved routes of administration which can result in overdose and death [see Overdosage (10)]. | |||
To reduce the abuse of CNS stimulants, including Vyvanse, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records, educate patients and their families about abuse and on proper storage and disposal of CNS stimulants, monitor for signs of abuse while on therapy, and re-evaluate the need for Vyvanse use. | |||
<u>Studies of Vyvanse in Drug Abusers</u> | |||
A randomized, double-blind, placebo-control, cross-over, abuse liability study in 38 patients with a history of drug abuse was conducted with single-doses of 50, 100, or 150 mg of Vyvanse, 40 mg of immediate-release d-amphetamine sulphate (a controlled II substance), and 200 mg of diethylpropion hydrochloride (a controlled IV substance). Vyvanse 100 mg produced significantly less "Drug Liking Effects" as measured by the Drug Rating Questionnaire-Subject score, compared to d-amphetamine 40 mg; and 150 mg of Vyvanse demonstrated similar "Drug-Liking Effects" compared to 40 mg of d-amphetamine and 200 mg of diethylpropion. | |||
Intravenous administration of 50 mg lisdexamfetamine dimesylate to individuals with a history of drug abuse produced positive subjective responses on scales measuring "Drug Liking", "Euphoria", "Amphetamine Effects", and "Benzedrine Effects" that were greater than placebo but less than those produced by an equivalent dose (20 mg) of intravenous d-amphetamine. | |||
===Dependence=== | |||
<i>Tolerance</i> | |||
Tolerance (a state of adaptation in which exposure to a drug results in a reduction of the drug's desired and/or undesired effects over time) may occur during the chronic therapy of CNS stimulants including Vyvanse. | |||
<i>Dependence</i> | |||
Physical dependence (a state of adaptation manifested by a withdrawal syndrome produced by abrupt cessation, rapid dose reduction, or administration of an antagonist) may occur in patients treated with CNS stimulants including Vyvanse. Withdrawal symptoms after abrupt cessation following prolonged high-dosage administration of CNS stimulants include extreme fatigue and depression. | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 418022579 | |||
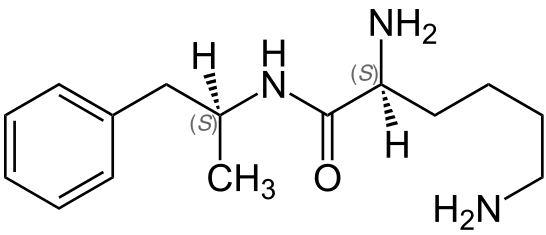
| IUPAC_name = (2''S'')-2,6-Diamino-''N''-[(2''S'')-1-phenylpropan-2-yl]hexanamide | |||
| image = Lisdexamfetamine-Structural Formula V.1.png | |||
| image2 = Lisdexamfetamine.gif | |||
<!--Clinical data--> | |||
| tradename = Tyvense, Elvanse, Venvanse, Vyvanse | |||
| Drugs.com = {{drugs.com|monograph|lisdexamfetamine-dimesylate}} | |||
| MedlinePlus = a607047 | |||
| pregnancy_AU = B3 | |||
| pregnancy_US = C | |||
| legal_US = Schedule II | |||
| licence_US = Lisdexamfetamine | |||
| legal_UK = Class B/Schedule II | |||
| legal_CA = Schedule I | |||
| legal_AU = S8 | |||
| routes_of_administration = Oral | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = 28% | |||
| metabolism = Gastro-intestinal (initial); Hepatic (extensively [[CYP2D6]]) after conversion to dextroamphetamine | |||
| elimination_half-life = < 1 hour ([[prodrug]] molecule), 10-13 hours (dextroamphetamine) | |||
| excretion = Renal: ~2% | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 608137-32-2 | |||
| ATC_prefix = N06 | |||
| ATC_suffix = BA12 | |||
| PubChem = 11597698 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB01255 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 9772458 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = H645GUL8KJ | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1201222 | |||
| synonyms = (2''S'')-2,6-diamino-''N''-[(1''S'')-1-methyl-2-phenylethyl]hexanamide <br><br> (2''S'')-2,6-Bis(azanyl)-''N''-[(2''S'')-1-phenylpropan-2-yl]hexanamide | |||
<!--Chemical data--> | |||
| C=15 | H=25 | N=3 | O=1 | |||
| molecular_weight = 263.378 g/mol | |||
| smiles = O=C(N[C@H](Cc1ccccc1)C)[C@@H](N)CCCCN | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C15H25N3O/c1-12(11-13-7-3-2-4-8-13)18-15(19)14(17)9-5-6-10-16/h2-4,7-8,12,14H,5-6,9-11,16-17H2,1H3,(H,18,19)/t12-,14-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = VOBHXZCDAVEXEY-JSGCOSHPSA-N | |||
}} | |||
|alcohol=Alcohol-Lisdexamfetamine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
}} | |||
Revision as of 21:26, 4 August 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Lisdexamfetamine is an {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Lisdexamfetamine FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lisdexamfetamine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lisdexamfetamine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Lisdexamfetamine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Lisdexamfetamine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Lisdexamfetamine in pediatric patients.
Contraindications
There is limited information regarding Lisdexamfetamine Contraindications in the drug label.
Warnings
There is limited information regarding Lisdexamfetamine Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Lisdexamfetamine Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Lisdexamfetamine Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Lisdexamfetamine Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Risk Summary
There are no adequate and well-controlled studies with Vyvanse in pregnant women. Adverse pregnancy outcomes, including premature delivery and low birth weight, have been seen in infants born to mothers dependent on amphetamines. Long-term neurochemical and behavioral effects have been reported in animal developmental studies using clinically relevant doses of amphetamine (d- or d,l-). Animal reproduction studies performed with lisdexamfetamine dimesylate in rats and rabbits showed no effects on embryofetal morphological development and survival. Vyvanse should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Amphetamines, such as Vyvanse, cause vasoconstriction and thereby may decrease placental perfusion. Infants born to amphetamine-dependent mothers have an increased risk of premature delivery and low birth weight. Monitor infants born to mothers taking amphetamines for symptoms of withdrawal such as feeding difficulties, irritability, agitation, and excessive drowsiness.
Human Data
Available data in women using amphetamines during pregnancy do not show a clear increased risk of major congenital malformations. Two case control studies of over a thousand patients in total exposed to amphetamines at different gestational ages did not show an increase in congenital abnormalities.
Animal Data
Lisdexamfetamine dimesylate had no apparent effects on embryofetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis at doses of up to 40 and 120 mg/kg/day, respectively. These doses are approximately 4 and 27 times, respectively, the maximum recommended human dose of 70 mg/day given to adolescents, on a mg/m2 body surface area basis.
A number of studies in rodents indicate that prenatal or early postnatal exposure to amphetamine (d- or d,l-) at doses similar to those used clinically can result in long-term neurochemical and behavioral alterations. Reported behavioral effects include learning and memory deficits, altered locomotor activity, and changes in sexual function.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Lisdexamfetamine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Lisdexamfetamine during labor and delivery.
Nursing Mothers
Amphetamines are excreted into human milk. Long-term neurodevelopmental effects on infants from amphetamine exposure are unknown. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness have been established in pediatric patients with ADHD ages 6 to 17 years [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. Safety and efficacy in pediatric patients below the age of 6 years have not been established.
Growth Suppression
Growth should be monitored during treatment with stimulants, including Vyvanse, and children who are not growing or gaining weight as expected may need to have their treatment interrupted
Juvenile Animal Data
Studies conducted in juvenile rats and dogs at clinically relevant doses showed growth suppression that partially or fully reversed in dogs and female rats but not in male rats after a four-week drug-free recovery period. A study was conducted in which juvenile rats received oral doses of 4, 10, or 40 mg/kg/day of lisdexamfetamine dimesylate from day 7 to day 63 of age. These doses are approximately 0.3, 0.7, and 3 times the maximum recommended human daily dose of 70 mg on a mg/m2 basis for a child. Dose-related decreases in food consumption, bodyweight gain, and crown-rump length were seen; after a four-week drug-free recovery period, bodyweights and crown-rump lengths had significantly recovered in females but were still substantially reduced in males. Time to vaginal opening was delayed in females at the highest dose, but there were no drug effects on fertility when the animals were mated beginning on day 85 of age. In a study in which juvenile dogs received lisdexamfetamine dimesylate for 6 months beginning at 10 weeks of age, decreased bodyweight gain was seen at all doses tested (2, 5, and 12 mg/kg/day, which are approximately 0.5, 1, and 3 times the maximum recommended human daily dose on a mg/m2 basis for a child). This effect partially or fully reversed during a four-week drug-free recovery period.
Geriatic Use
Clinical studies of Vyvanse did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should start at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
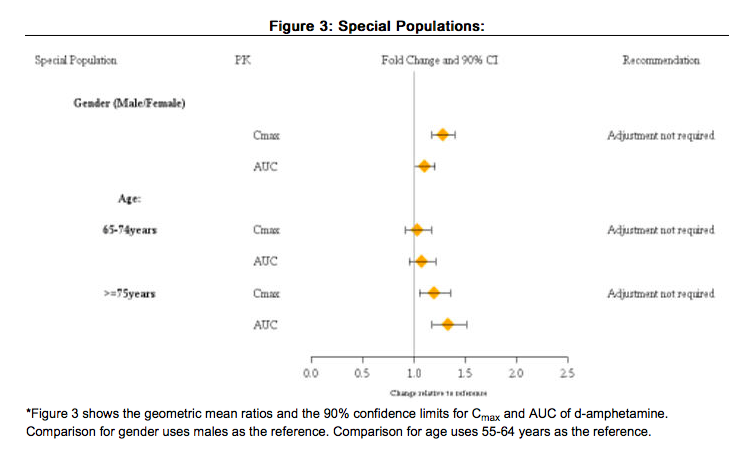
Gender
There is no FDA guidance on the use of Lisdexamfetamine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Lisdexamfetamine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Lisdexamfetamine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Lisdexamfetamine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Lisdexamfetamine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Lisdexamfetamine in patients who are immunocompromised.
Special Populations
Administration and Monitoring
Administration
General Instructions for Use
Take Vyvanse by mouth in the morning with or without food; avoid afternoon doses because of the potential for insomnia. Vyvanse may be administered in one of the following ways:
- Swallow Vyvanse capsules whole, or
- Open capsules, empty and mix the entire contents with yogurt, water, or orange juice. If the contents of the capsule include any compacted powder, a spoon may be used to break apart the powder. The contents should be mixed until completely dispersed. Consume the entire mixture immediately. It should not be stored. The active ingredient dissolves completely once dispersed; however, a film containing the inactive ingredients may remain in the glass or container once the mixture is consumed. Do not take anything less than one capsule per day, and a single capsule should not be divided.
Monitoring
FDA Package Insert for Lisdexamfetamine contains no information regarding drug monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
Consult with a Certified Poison Control Center (1-800-222-1222) for up-to-date guidance and advice for treatment of overdosage. Individual patient response to amphetamines varies widely. Toxic symptoms may occur idiosyncratically at low doses. Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Other reactions include arrhythmias, hypertension or hypotension, circulatory collapse, nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
DRUG ABUSE AND DEPENDENCE
Controlled Substance
Vyvanse contains lisdexamfetamine, a prodrug of amphetamine, a Schedule II controlled substance.
Abuse
CNS stimulants, including Vyvanse, other amphetamines, and methylphenidate-containing products have a high potential for abuse. Abuse is characterized by impaired control over drug use, compulsive use, continued use despite harm, and craving. Signs and symptoms of CNS stimulant abuse may include increased heart rate, respiratory rate, blood pressure, and/or sweating, dilated pupils, hyperactivity, restlessness, insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and/or abdominal pain. Anxiety, psychosis, hostility, aggression, suicidal or homicidal ideation have also been seen. Abusers of CNS stimulants may chew, snort, inject, or use other unapproved routes of administration which can result in overdose and death [see Overdosage (10)]. To reduce the abuse of CNS stimulants, including Vyvanse, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records, educate patients and their families about abuse and on proper storage and disposal of CNS stimulants, monitor for signs of abuse while on therapy, and re-evaluate the need for Vyvanse use.
Studies of Vyvanse in Drug Abusers
A randomized, double-blind, placebo-control, cross-over, abuse liability study in 38 patients with a history of drug abuse was conducted with single-doses of 50, 100, or 150 mg of Vyvanse, 40 mg of immediate-release d-amphetamine sulphate (a controlled II substance), and 200 mg of diethylpropion hydrochloride (a controlled IV substance). Vyvanse 100 mg produced significantly less "Drug Liking Effects" as measured by the Drug Rating Questionnaire-Subject score, compared to d-amphetamine 40 mg; and 150 mg of Vyvanse demonstrated similar "Drug-Liking Effects" compared to 40 mg of d-amphetamine and 200 mg of diethylpropion. Intravenous administration of 50 mg lisdexamfetamine dimesylate to individuals with a history of drug abuse produced positive subjective responses on scales measuring "Drug Liking", "Euphoria", "Amphetamine Effects", and "Benzedrine Effects" that were greater than placebo but less than those produced by an equivalent dose (20 mg) of intravenous d-amphetamine.
Dependence
Tolerance
Tolerance (a state of adaptation in which exposure to a drug results in a reduction of the drug's desired and/or undesired effects over time) may occur during the chronic therapy of CNS stimulants including Vyvanse.
Dependence
Physical dependence (a state of adaptation manifested by a withdrawal syndrome produced by abrupt cessation, rapid dose reduction, or administration of an antagonist) may occur in patients treated with CNS stimulants including Vyvanse. Withdrawal symptoms after abrupt cessation following prolonged high-dosage administration of CNS stimulants include extreme fatigue and depression.
Pharmacology
Mechanism of Action
There is limited information regarding Lisdexamfetamine Mechanism of Action in the drug label.
Structure
There is limited information regarding Lisdexamfetamine Structure in the drug label.
Pharmacodynamics
There is limited information regarding Lisdexamfetamine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Lisdexamfetamine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Lisdexamfetamine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Lisdexamfetamine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Lisdexamfetamine How Supplied in the drug label.
Storage
There is limited information regarding Lisdexamfetamine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Lisdexamfetamine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Lisdexamfetamine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Lisdexamfetamine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Lisdexamfetamine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Lisdexamfetamine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Lisdexamfetamine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.