Linitis plastica pathophysiology: Difference between revisions
No edit summary |
|||
| (36 intermediate revisions by 3 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Linitis plastica}} | {{Linitis plastica}} | ||
{{CMG}}{{AE}} {{ | {{CMG}};{{AE}}{{HM}} | ||
==Overview== | ==Overview== | ||
It is thought that linitis plastica is generally a sporadic disease that may be inherited as a result of abnormal molecular pathways (Beta catenin/Wnt signaling) that causes defective [[Cell adhesion|intracellular adhesions]]. | |||
==Pathophysiology== | ==Pathophysiology== | ||
* | *In diffuse type [[Stomach cancer|gastric cancer]], the cells experience a loss of expression for [[Cell adhesion|cell adhesion protein]] [[Cadherin|E-cadherin]].<ref name="pmid14630673">{{cite journal |vauthors=Graziano F, Humar B, Guilford P |title=The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice |journal=Ann. Oncol. |volume=14 |issue=12 |pages=1705–13 |year=2003 |pmid=14630673 |doi= |url=}}</ref> | ||
* | *The [[Cadherin|E-cadherin]] gene ([[CDH1 (gene)|CDH1]]) codes for a transmembrane cellular adhesion protein that provides a tail that adheres to other cells. | ||
*The tail interacts with catenins in the neighbouring cell and forms a cell to cell complex in what is known as Beta catenin/Wnt signaling. | |||
*The loss of these cellular interactions leads to the formation of cancerous cells that trigger a stromal reaction leading to diffuse schirrous (fibrous) changes. | |||
* | |||
| | ==Genetics== | ||
*Linitis plastica is transmitted in an [[autosomal dominant]] pattern in hereditary diffuse gastric cancer.<ref name="pmid9537325">{{cite journal |vauthors=Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE |title=E-cadherin germline mutations in familial gastric cancer |journal=Nature |volume=392 |issue=6674 |pages=402–5 |year=1998 |pmid=9537325 |doi=10.1038/32918 |url=}}</ref><ref name="pmid18788075">{{cite journal |vauthors=Barber M, Murrell A, Ito Y, Maia AT, Hyland S, Oliveira C, Save V, Carneiro F, Paterson AL, Grehan N, Dwerryhouse S, Lao-Sirieix P, Caldas C, Fitzgerald RC |title=Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer |journal=J. Pathol. |volume=216 |issue=3 |pages=295–306 |year=2008 |pmid=18788075 |doi=10.1002/path.2426 |url=}}</ref><ref name="pmid19269290">{{cite journal |vauthors=Oliveira C, Sousa S, Pinheiro H, Karam R, Bordeira-Carriço R, Senz J, Kaurah P, Carvalho J, Pereira R, Gusmão L, Wen X, Cipriano MA, Yokota J, Carneiro F, Huntsman D, Seruca R |title=Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression |journal=Gastroenterology |volume=136 |issue=7 |pages=2137–48 |year=2009 |pmid=19269290 |doi=10.1053/j.gastro.2009.02.065 |url=}}</ref> | |||
*Genes involved in the pathogenesis of linitis plastica include [[CDH1 (gene)|CDH1 gene]], located on chromosome 16q22.1, which codes for the [[Cadherin|E-cadherin]] protein. | |||
*The development of linitis plastica is the result of germline truncating mutations spread over several [[Exon|exons]]. | |||
*Consequently, the second [[allele]] coding for [[Cadherin|E-cadherin]] is inactivated in a two-hit theoretical manner. | |||
*Mutations include: | |||
**[[Promoter]] hypermethylation | |||
**[[Loss of heterozygosity]] | |||
**Silencing mutation | |||
}}</ref> | ==Associated conditions== | ||
* | *Women with diffuse type gastric cancer are also at an increased risk of [[Breast cancer|lobular breast cancer]]. | ||
*Susceptibility to diffuse [[Stomach cancer|gastric cancer]] may also be linked to inherited [[polymorphisms]] in the [[PSCA (gene)|PSCA]] ([[prostate]] stem cell antigen) gene, which is possibly involved in regulating gastric [[Epithelial cells|epithelial cell]] [[Cell growth|proliferation]].<ref name="pmid18488030">{{cite journal |vauthors=Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F, Kato S, Matsukura N, Matsuda N, Nakamura T, Hyodo I, Nishina T, Yasui W, Hirose H, Hayashi M, Toshiro E, Ohnami S, Sekine A, Sato Y, Totsuka H, Ando M, Takemura R, Takahashi Y, Ohdaira M, Aoki K, Honmyo I, Chiku S, Aoyagi K, Sasaki H, Ohnami S, Yanagihara K, Yoon KA, Kook MC, Lee YS, Park SR, Kim CG, Choi IJ, Yoshida T, Nakamura Y, Hirohashi S |title=Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer |journal=Nat. Genet. |volume=40 |issue=6 |pages=730–40 |year=2008 |pmid=18488030 |doi=10.1038/ng.152 |url=}}</ref> | |||
==Gross Pathology== | |||
*On gross pathology, a thickened, rigid, and leather-like stomach wall is a characteristic finding of linitis plastica. | |||
*Linitis plastica may appear as [[Plaque|plaques]] of [[fibrosis]], which give the appearance of a segmental infiltration with a lack of distensibility. | |||
[[Image:lp.jpg|thumb|center|500px|Shown is diffuse cancer of the stomach, giving a "leather bottle appearance".Source: commons.wikimedia.org by http://en.wikipedia.org/wiki/User:Samir_%28The_Scope%29 - http://en.wikipedia.org/wiki/Image:Linitis_plastica.jpg, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=1649074]] | |||
==Microscopic Pathology== | |||
On microscopic histopathological analysis, tumor cells are seen invading surrounding tissue, with no glandular formation and abundant [[mucin]] that push the [[Cell nucleus|nucleus]] to one side producing what is known as a [[signet ring cell]]. [[Signet cell|Signet ring cell]]<nowiki/>s are a characteristic finding of linitis plastica.<ref name="pmid19855261">{{cite journal |vauthors=Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C |title=Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation |journal=Ann. Surg. |volume=250 |issue=6 |pages=878–87 |year=2009 |pmid=19855261 |doi=10.1097/SLA.0b013e3181b21c7b |url=}}</ref> | |||
[[Image:sc.jpg|thumb|center|500px|Arrow indicates a signet ring cell characteristic of linitis plastica. Source:commons.wikimedia.org by Ed Uthman from Houston, TX, USA - Signet Ring CellsUploaded by CFCF, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=30103742 ]] | |||
==References== | ==References== | ||
| Line 31: | Line 43: | ||
[[Category:Gastroenterology]] | [[Category:Gastroenterology]] | ||
[[Category:Disease]] | [[Category:Disease]] | ||
{{WikiDoc Help Menu}} | {{WikiDoc Help Menu}} | ||
{{WikiDoc Sources}} | {{WikiDoc Sources}} | ||
Latest revision as of 19:32, 16 January 2018
|
Linitis plastica Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Linitis plastica pathophysiology On the Web |
|
American Roentgen Ray Society Images of Linitis plastica pathophysiology |
|
Risk calculators and risk factors for Linitis plastica pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Hadeel Maksoud M.D.[2]
Overview
It is thought that linitis plastica is generally a sporadic disease that may be inherited as a result of abnormal molecular pathways (Beta catenin/Wnt signaling) that causes defective intracellular adhesions.
Pathophysiology
- In diffuse type gastric cancer, the cells experience a loss of expression for cell adhesion protein E-cadherin.[1]
- The E-cadherin gene (CDH1) codes for a transmembrane cellular adhesion protein that provides a tail that adheres to other cells.
- The tail interacts with catenins in the neighbouring cell and forms a cell to cell complex in what is known as Beta catenin/Wnt signaling.
- The loss of these cellular interactions leads to the formation of cancerous cells that trigger a stromal reaction leading to diffuse schirrous (fibrous) changes.
Genetics
- Linitis plastica is transmitted in an autosomal dominant pattern in hereditary diffuse gastric cancer.[2][3][4]
- Genes involved in the pathogenesis of linitis plastica include CDH1 gene, located on chromosome 16q22.1, which codes for the E-cadherin protein.
- The development of linitis plastica is the result of germline truncating mutations spread over several exons.
- Consequently, the second allele coding for E-cadherin is inactivated in a two-hit theoretical manner.
- Mutations include:
- Promoter hypermethylation
- Loss of heterozygosity
- Silencing mutation
Associated conditions
- Women with diffuse type gastric cancer are also at an increased risk of lobular breast cancer.
- Susceptibility to diffuse gastric cancer may also be linked to inherited polymorphisms in the PSCA (prostate stem cell antigen) gene, which is possibly involved in regulating gastric epithelial cell proliferation.[5]
Gross Pathology
- On gross pathology, a thickened, rigid, and leather-like stomach wall is a characteristic finding of linitis plastica.
- Linitis plastica may appear as plaques of fibrosis, which give the appearance of a segmental infiltration with a lack of distensibility.
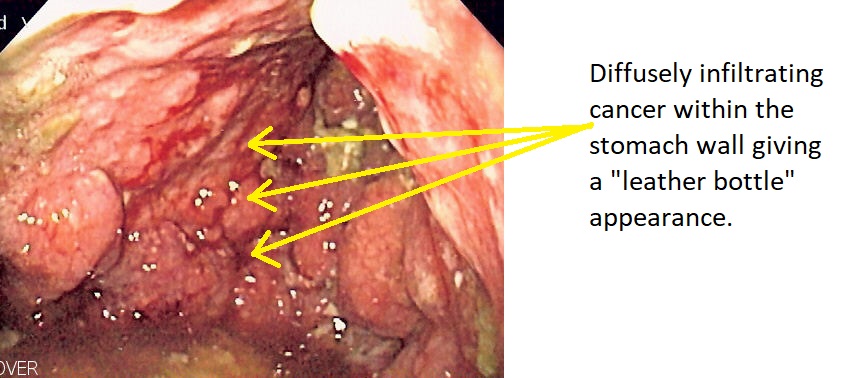
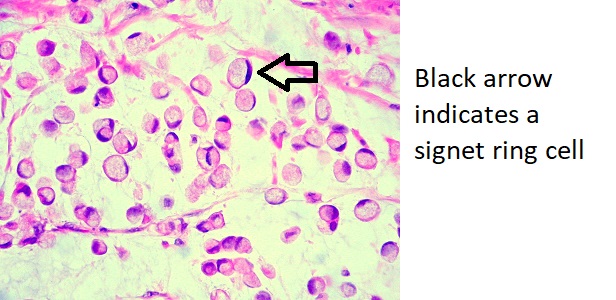
Microscopic Pathology
On microscopic histopathological analysis, tumor cells are seen invading surrounding tissue, with no glandular formation and abundant mucin that push the nucleus to one side producing what is known as a signet ring cell. Signet ring cells are a characteristic finding of linitis plastica.[6]
References
- ↑ Graziano F, Humar B, Guilford P (2003). "The role of the E-cadherin gene (CDH1) in diffuse gastric cancer susceptibility: from the laboratory to clinical practice". Ann. Oncol. 14 (12): 1705–13. PMID 14630673.
- ↑ Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE (1998). "E-cadherin germline mutations in familial gastric cancer". Nature. 392 (6674): 402–5. doi:10.1038/32918. PMID 9537325.
- ↑ Barber M, Murrell A, Ito Y, Maia AT, Hyland S, Oliveira C, Save V, Carneiro F, Paterson AL, Grehan N, Dwerryhouse S, Lao-Sirieix P, Caldas C, Fitzgerald RC (2008). "Mechanisms and sequelae of E-cadherin silencing in hereditary diffuse gastric cancer". J. Pathol. 216 (3): 295–306. doi:10.1002/path.2426. PMID 18788075.
- ↑ Oliveira C, Sousa S, Pinheiro H, Karam R, Bordeira-Carriço R, Senz J, Kaurah P, Carvalho J, Pereira R, Gusmão L, Wen X, Cipriano MA, Yokota J, Carneiro F, Huntsman D, Seruca R (2009). "Quantification of epigenetic and genetic 2nd hits in CDH1 during hereditary diffuse gastric cancer syndrome progression". Gastroenterology. 136 (7): 2137–48. doi:10.1053/j.gastro.2009.02.065. PMID 19269290.
- ↑ Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T, Matsuno Y, Saito D, Sugimura H, Tanioka F, Kato S, Matsukura N, Matsuda N, Nakamura T, Hyodo I, Nishina T, Yasui W, Hirose H, Hayashi M, Toshiro E, Ohnami S, Sekine A, Sato Y, Totsuka H, Ando M, Takemura R, Takahashi Y, Ohdaira M, Aoki K, Honmyo I, Chiku S, Aoyagi K, Sasaki H, Ohnami S, Yanagihara K, Yoon KA, Kook MC, Lee YS, Park SR, Kim CG, Choi IJ, Yoshida T, Nakamura Y, Hirohashi S (2008). "Genetic variation in PSCA is associated with susceptibility to diffuse-type gastric cancer". Nat. Genet. 40 (6): 730–40. doi:10.1038/ng.152. PMID 18488030.
- ↑ Piessen G, Messager M, Leteurtre E, Jean-Pierre T, Mariette C (2009). "Signet ring cell histology is an independent predictor of poor prognosis in gastric adenocarcinoma regardless of tumoral clinical presentation". Ann. Surg. 250 (6): 878–87. doi:10.1097/SLA.0b013e3181b21c7b. PMID 19855261.