Ipilimumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Immune-mediated adverse reactions
See full prescribing information for complete Boxed Warning.
Ipilimumab can result in severe and fatal immune-mediated adverse reactions due to T-cell activation and proliferation. These immune-mediated reactions may involve any organ system; however, the most common severe immune-mediated adverse reactions are enterocolitis, hepatitis, dermatitis (including toxic epidermal necrolysis), neuropathy, and endocrinopathy. The majority of these immune-mediated reactions initially manifested during treatment; however, a minority occurred weeks to months after discontinuation of ipilimumab.
Permanently discontinue ipilimumab and initiate systemic high-dose corticosteroid therapy for severe immune-mediated reactions. Assess patients for signs and symptoms of enterocolitis, dermatitis, neuropathy, and endocrinopathy and evaluate clinical chemistries including liver function tests and thyroid function tests at baseline and before each dose. |
Overview
Ipilimumab is an monoclonal antibody that is FDA approved for the treatment of unresectable or metastatic melanoma. There is a Black Box Warning for this drug as shown here. Common adverse reactions include pruritus, colitis, fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Ipilimumab is indicated for the treatment of unresectable or metastatic melanoma.
- Dosage: 3 mg/kg administered intravenously over 90 minutes every 3 weeks for a total of 4 doses.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of ipilimumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of ipilimumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ipilimumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of ipilimumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of ipilimumab in pediatric patients.
Contraindications
None
Warnings
|
Immune-mediated adverse reactions
See full prescribing information for complete Boxed Warning.
Ipilimumab can result in severe and fatal immune-mediated adverse reactions due to T-cell activation and proliferation. These immune-mediated reactions may involve any organ system; however, the most common severe immune-mediated adverse reactions are enterocolitis, hepatitis, dermatitis (including toxic epidermal necrolysis), neuropathy, and endocrinopathy. The majority of these immune-mediated reactions initially manifested during treatment; however, a minority occurred weeks to months after discontinuation of ipilimumab.
Permanently discontinue ipilimumab and initiate systemic high-dose corticosteroid therapy for severe immune-mediated reactions. Assess patients for signs and symptoms of enterocolitis, dermatitis, neuropathy, and endocrinopathy and evaluate clinical chemistries including liver function tests and thyroid function tests at baseline and before each dose. |
Ipilimumab can result in severe and fatal immune-mediated reactions due to T-cell activation and proliferation.
Immune-mediated Enterocolitis
- In Study 1, severe, life-threatening, or fatal (diarrhea of 7 or more stools above baseline, fever, ileus, peritoneal signs; Grade 3–5) immune-mediated enterocolitis occurred in 34 (7%) ipilimumab-treated patients, and moderate (diarrhea with up to 6 stools above baseline, abdominal pain, mucus or blood in stool; Grade 2) enterocolitis occurred in 28 (5%) ipilimumab-treated patients. Across all ipilimumab-treated patients (n=511), 5 (1%) patients developed intestinal perforation, 4 (0.8%) patients died as a result of complications, and 26 (5%) patients were hospitalized for severe enterocolitis.
- The median time to onset was 7.4 weeks (range: 1.6–13.4) and 6.3 weeks (range: 0.3–18.9) after the initiation of ipilimumab for patients with Grade 3–5 enterocolitis and with Grade 2 enterocolitis, respectively.
- Twenty-nine patients (85%) with Grade 3–5 enterocolitis were treated with high-dose (≥40 mg prednisone equivalent per day) corticosteroids, with a median dose of 80 mg/day of prednisone or equivalent; the median duration of treatment was 2.3 weeks (ranging up to 13.9 weeks) followed by corticosteroid taper. Of the 28 patients with moderate enterocolitis, 46% were not treated with systemic corticosteroids, 29% were treated with <40 mg prednisone or equivalent per day for a median duration of 5.1 weeks, and 25% were treated with high-dose corticosteroids for a median duration of 10 days prior to corticosteroid taper. Infliximab was administered to 5 of the 62 patients (8%) with moderate, severe, or life-threatening immune-mediated enterocolitis following inadequate response to corticosteroids.
- Of the 34 patients with Grade 3–5 enterocolitis, 74% experienced complete resolution, 3% experienced improvement to Grade 2 severity, and 24% did not improve. Among the 28 patients with Grade 2 enterocolitis, 79% experienced complete resolution, 11% improved, and 11% did not improve.
- Monitor patients for signs and symptoms of enterocolitis (such as diarrhea, abdominal pain, mucus or blood in stool, with or without fever) and of bowel perforation (such as peritoneal signs and ileus). In symptomatic patients, rule out infectious etiologies and consider endoscopic evaluation for persistent or severe symptoms.
- Permanently discontinue ipilimumab in patients with severe enterocolitis and initiate systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or equivalent. Upon improvement to Grade 1 or less, initiate corticosteroid taper and continue to taper over at least 1 month. In clinical trials, rapid corticosteroid tapering resulted in recurrence or worsening symptoms of enterocolitis in some patients.
- Withhold ipilimumab dosing for moderate enterocolitis; administer anti-diarrheal treatment and, if persistent for more than 1 week, initiate systemic corticosteroids at a dose of 0.5 mg/kg/day prednisone or equivalent.
Immune-mediated Hepatitis
- In Study 1, severe, life-threatening, or fatal hepatotoxicity (AST or ALT elevations of more than 5 times the upper limit of normal or total bilirubin elevations more than 3 times the upper limit of normal; Grade 3–5) occurred in 8 (2%) ipilimumab-treated patients, with fatal hepatic failure in 0.2% and hospitalization in 0.4% of ipilimumab-treated patients. An additional 13 (2.5%) patients experienced moderate hepatotoxicity manifested by liver function test abnormalities (AST or ALT elevations of more than 2.5 times but not more than 5 times the upper limit of normal or total bilirubin elevation of more than 1.5 times but not more than 3 times the upper limit of normal; Grade 2). The underlying pathology was not ascertained in all patients but in some instances included immune-mediated hepatitis. There were insufficient numbers of patients with biopsy-proven hepatitis to characterize the clinical course of this event.
- Monitor liver function tests (hepatic transaminase and bilirubin levels) and assess patients for signs and symptoms of hepatotoxicity before each dose of ipilimumab. In patients with hepatotoxicity, rule out infectious or malignant causes and increase frequency of liver function test monitoring until resolution.
- Permanently discontinue ipilimumab in patients with Grade 3–5 hepatotoxicity and administer systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or equivalent. When liver function tests show sustained improvement or return to baseline, initiate corticosteroid tapering and continue to taper over 1 month. Across the clinical development program for ipilimumab, mycophenolate treatment has been administered in patients who have persistent severe hepatitis despite high-dose corticosteroids. Withhold ipilimumab in patients with Grade 2 hepatotoxicity.
Concurrent Administration with Vemurafenib
- In a dose-finding trial, Grade 3 increases in transaminases with or without concomitant increases in total bilirubin occurred in 6 of 10 patients who received concurrent ipilimumab (3 mg/kg) and vemurafenib (960 mg BID or 720 mg BID).
Immune-mediated Dermatitis
- In Study 1, severe, life-threatening, or fatal immune-mediated dermatitis (eg, Stevens-Johnson syndrome, toxic epidermal necrolysis, or rash complicated by full thickness dermal ulceration, or necrotic, bullous, or hemorrhagic manifestations; Grade 3–5) occurred in 13 (2.5%) ipilimumab-treated patients. One (0.2%) patient died as a result of toxic epidermal necrolysis and one additional patient required hospitalization for severe dermatitis. There were 63 (12%) patients with moderate (Grade 2) dermatitis.
- The median time to onset of moderate, severe, or life-threatening immune-mediated dermatitis was 3.1 weeks and ranged up to 17.3 weeks from the initiation of ipilimumab.
- Seven (54%) ipilimumab-treated patients with severe dermatitis received high-dose corticosteroids (median dose 60 mg prednisone/day or equivalent) for up to 14.9 weeks followed by corticosteroid taper. Of these 7 patients, 6 had complete resolution; time to resolution ranged up to 15.6 weeks.
- Of the 63 patients with moderate dermatitis, 25 (40%) were treated with systemic corticosteroids (median of 60 mg/day of prednisone or equivalent) for a median of 2.1 weeks, 7 (11%) were treated with only topical corticosteroids, and 31 (49%) did not receive systemic or topical corticosteroids. Forty-four (70%) patients with moderate dermatitis were reported to have complete resolution, 7 (11%) improved to mild (Grade 1) severity, and 12 (19%) had no reported improvement.
- Monitor patients for signs and symptoms of dermatitis such as rash and pruritus. Unless an alternate etiology has been identified, signs or symptoms of dermatitis should be considered immune-mediated.
- Permanently discontinue ipilimumab in patients with Stevens-Johnson syndrome, toxic epidermal necrolysis, or rash complicated by full thickness dermal ulceration, or necrotic, bullous, or hemorrhagic manifestations. Administer systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or equivalent. When dermatitis is controlled, corticosteroid tapering should occur over a period of at least 1 month. Withhold ipilimumab dosing in patients with moderate to severe signs and symptoms.
- For mild to moderate dermatitis, such as localized rash and pruritus, treat symptomatically. Administer topical or systemic corticosteroids if there is no improvement of symptoms within 1 week.
Immune-mediated Neuropathies
- In Study 1, 1 case of fatal Guillain-Barré syndrome and 1 case of severe (Grade 3) peripheral motor neuropathy were reported. Across the clinical development program of ipilimumab, myasthenia gravis and additional cases of Guillain-Barré syndrome have been reported.
- Monitor for symptoms of motor or sensory neuropathy such as unilateral or bilateral weakness, sensory alterations, or paresthesia. Permanently discontinue ipilimumab in patients with severe neuropathy (interfering with daily activities) such as Guillain-Barré-like syndromes. Institute medical intervention as appropriate for management of severe neuropathy. Consider initiation of systemic corticosteroids at a dose of 1 to 2 mg/kg/day prednisone or equivalent for severe neuropathies. Withhold ipilimumab dosing in patients with moderate neuropathy (not interfering with daily activities).
Immune-mediated Endocrinopathies
- In Study 1, severe to life-threatening immune-mediated endocrinopathies (requiring hospitalization, urgent medical intervention, or interfering with activities of daily living; Grade 3–4) occurred in 9 (1.8%) ipilimumab-treated patients. All 9 patients had hypopituitarism and some had additional concomitant endocrinopathies such as adrenal insufficiency, hypogonadism, and hypothyroidism. Six of the 9 patients were hospitalized for severe endocrinopathies. Moderate endocrinopathy (requiring hormone replacement or medical intervention; Grade 2) occurred in 12 (2.3%) patients and consisted of hypothyroidism, adrenal insufficiency, hypopituitarism, and 1 case each of hyperthyroidism and Cushing’s syndrome. The median time to onset of moderate to severe immune-mediated endocrinopathy was 11 weeks and ranged up to 19.3 weeks after the initiation of ipilimumab.
- Of the 21 patients with moderate to life-threatening endocrinopathy, 17 patients required long-term hormone replacement therapy including, most commonly, adrenal hormones (n=10) and thyroid hormones (n=13).
- Monitor patients for clinical signs and symptoms of hypophysitis, adrenal insufficiency (including adrenal crisis), and hyper- or hypothyroidism. Patients may present with fatigue, headache, mental status changes, abdominal pain, unusual bowel habits, and hypotension, or nonspecific symptoms which may resemble other causes such as brain metastasis or underlying disease. Unless an alternate etiology has been identified, signs or symptoms of endocrinopathies should be considered immune-mediated.
- Monitor thyroid function tests and clinical chemistries at the start of treatment, before each dose, and as clinically indicated based on symptoms. In a limited number of patients, hypophysitis was diagnosed by imaging studies through enlargement of the pituitary gland.
- Withhold ipilimumab dosing in symptomatic patients. Initiate systemic corticosteroids at a dose of 1 to 2 mg/kg/day of prednisone or equivalent, and initiate appropriate hormone replacement therapy.
Other Immune-mediated Adverse Reactions, Including Ocular Manifestations
- The following clinically significant immune-mediated adverse reactions were seen in less than 1% of ipilimumab-treated patients in Study 1: nephritis, pneumonitis, meningitis, pericarditis, uveitis, iritis, and hemolytic anemia.
- Across the clinical development program for ipilimumab, the following likely immune-mediated adverse reactions were also reported with less than 1% incidence: myocarditis, angiopathy, temporal arteritis, vasculitis, polymyalgia rheumatica, conjunctivitis, blepharitis, episcleritis, scleritis, leukocytoclastic vasculitis, erythema multiforme, psoriasis, pancreatitis, arthritis, autoimmune thyroiditis, sarcoidosis, neurosensory hypoacusis, autoimmune central neuropathy (encephalitis), myositis, polymyositis, and ocular myositis.
- Permanently discontinue ipilimumab for clinically significant or severe immune-mediated adverse reactions. Initiate systemic corticosteroids at a dose of 1 to 2 mg/kg/day prednisone or equivalent for severe immune-mediated adverse reactions.
- Administer corticosteroid eye drops to patients who develop uveitis, iritis, or episcleritis. Permanently discontinue ipilimumab for immune-mediated ocular disease that is unresponsive to local immunosuppressive therapy.
Adverse Reactions
Clinical Trials Experience
- Immune-mediated enterocolitis
- Immune-mediated hepatitis
- Immune-mediated dermatitis
- Immune-mediated neuropathies
- Immune-mediated endocrinopathies
- Other immune-mediated adverse reactions, including ocular manifestations
Postmarketing Experience
There is limited information regarding Ipilimumab Postmarketing Experience in the drug label.
Drug Interactions
No formal pharmacokinetic drug interaction studies have been conducted with ipilimumab.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled studies of ipilimumab in pregnant women. Use ipilimumab during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In a combined study of embryo-fetal and peri-postnatal development, pregnant cynomolgus monkeys received ipilimumab every 3 weeks from the onset of organogenesis in the first trimester through parturition, at exposure levels either 2.6 or 7.2 times higher by AUC than the exposures at the clinical dose of 3 mg/kg of ipilimumab. No treatment-related adverse effects on reproduction were detected during the first two trimesters of pregnancy. Beginning in the third trimester, the ipilimumab treated groups experienced higher incidences of severe toxicities including abortion, stillbirth, premature delivery (with corresponding lower birth weight), and higher incidences of infant mortality in a dose-related manner compared to controls.
Human IgG1 is known to cross the placental barrier and ipilimumab is an IgG1; therefore, ipilimumab has the potential to be transmitted from the mother to the developing fetus.
Pregnancy Category (AUS): C
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ipilimumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ipilimumab during labor and delivery.
Nursing Mothers
It is not known whether ipilimumab is secreted in human milk. In monkeys treated at dose levels resulting in exposures 2.6 and 7.2 times higher than those in humans at the recommended dose, ipilimumab was present in milk at concentrations of 0.1 and 0.4 mcg/mL, representing a ratio of up to 0.3% of the serum concentration of the drug. Because many drugs are secreted in human milk and because of the potential for serious adverse reactions in nursing infants from ipilimumab, a decision should be made whether to discontinue nursing or to discontinue ipilimumab, taking into account the importance of ipilimumab to the mother.
Pediatric Use
Safety and effectiveness of ipilimumab have not been established in pediatric patients.
Geriatic Use
Of the 511 patients treated with ipilimumab at 3 mg/kg, 28% were 65 years and over. No overall differences in safety or efficacy were reported between the elderly patients (65 years and over) and younger patients (less than 65 years).
Gender
There is no FDA guidance on the use of Ipilimumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ipilimumab with respect to specific racial populations.
Renal Impairment
No dose adjustment is needed for patients with renal impairment.
Hepatic Impairment
No dose adjustment is needed for patients with mild hepatic impairment (total bilirubin [TB] >1.0 × to 1.5 × the upper limit of normal [ULN] or AST >ULN). Ipilimumab has not been studied in patients with moderate (TB >1.5 × to 3.0 × ULN and any AST) or severe (TB >3 × ULN and any AST) hepatic impairment.
Females of Reproductive Potential and Males
Fertility studies have not been performed with ipilimumab.
Immunocompromised Patients
There is no FDA guidance one the use of Ipilimumab in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Ipilimumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ipilimumab and IV administrations.
Overdosage
There is no information on overdosage with ipilimumab
Pharmacology
Ipilimumab?
| |
| Therapeutic monoclonal antibody | |
| Source | u |
| Target | CTLA-4 |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 148634.914 g/mol |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
|
| Pregnancy cat. |
C(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | IV |
Mechanism of Action
CTLA-4 is a negative regulator of T-cell activation. Ipilimumab binds to CTLA-4 and blocks the interaction of CTLA-4 with its ligands, CD80/CD86. Blockade of CTLA-4 has been shown to augment T-cell activation and proliferation. The mechanism of action of ipilimumab’s effect in patients with melanoma is indirect, possibly through T-cell mediated anti-tumor immune responses.
Structure
Is a recombinant, human monoclonal antibody that binds to the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). Ipilimumab is an IgG1 kappa immunoglobulin with an approximate molecular weight of 148 kDa. Ipilimumab is produced in mammalian (Chinese hamster ovary) cell culture.
Pharmacodynamics
There is limited information regarding pharmacodynamics of ipilimumab
Pharmacokinetics
The pharmacokinetics of ipilimumab were studied in 785 patients with unresectable or metastatic melanoma who received doses of 0.3, 3, or 10 mg/kg once every 3 weeks for 4 doses. Peak concentration (Cmax), trough concentration (Cmin), and area under the plasma concentration versus time curve (AUC) of ipilimumab increased dose proportionally within the dose range examined. Upon repeated dosing every 3 weeks, the clearance (CL) of ipilimumab was found to be time-invariant, and systemic accumulation was 1.5-fold or less. Steady-state concentrations of ipilimumab were reached by the third dose; the mean Cmin at steady-state was 19.4 mcg/mL following repeated doses of 3 mg/kg. The mean value (% coefficient of variation) generated through population pharmacokinetic analysis for the terminal half-life (t1/2) was 15.4 days (34%) and for CL was 16.8 mL/h (38%).
Specific Populations
The effects of various covariates on the pharmacokinetics of ipilimumab were assessed in population pharmacokinetic analyses. The CL of ipilimumab increased with increasing body weight; however, no dose adjustment is recommended for body weight after administration on a mg/kg basis. The following factors had no clinically important effect on the CL of ipilimumab: age (range: 23–88 years), gender, performance status, renal impairment, mild hepatic impairment, previous cancer therapy, and baseline lactate dehydrogenase (LDH) levels. The effect of race was not examined due to limited data available in non-Caucasian ethnic groups.
Renal Impairment
The effect of renal impairment on the CL of ipilimumab was evaluated in patients with mild (GFR <90 and ≥60 mL/min/1.73 m2; n=349), moderate (GFR <60 and ≥30 mL/min/1.73 m2; n=82), or severe (GFR <30 and ≥15 mL/min/1.73 m2; n=4) renal impairment compared to patients with normal renal function (GFR ≥90 mL/min/1.73 m2; n=350) in population pharmacokinetic analyses. No clinically important differences in the CL of ipilimumab were found between patients with renal impairment and patients with normal renal function.
Hepatic Impairment
The effect of hepatic impairment on the CL of ipilimumab was evaluated in patients with mild hepatic impairment (TB 1.0 × to 1.5 × ULN or AST >ULN as defined using the National Cancer Institute criteria of hepatic dysfunction; n=76) compared to patients with normal hepatic function (TB and AST ≤ULN; n=708) in the population pharmacokinetic analyses. No clinically important differences in the CL of ipilimumab were found between patients with mild hepatic impairment and normal hepatic function. ipilimumab has not been studied in patients with moderate (TB >1.5 × to 3 × ULN and any AST) or severe hepatic impairment (TB >3 × ULN and any AST).
Nonclinical Toxicology
Carcinogenesis
The carcinogenic potential of ipilimumab has not been evaluated in long-term animal studies.
Mutagenesis
The genotoxic potential of ipilimumab has not been evaluated.
Animal Toxicology and/or Pharmacology
- In addition to the severe findings of abortion, stillbirths, and postnatal deaths observed in pregnant cynomolgus monkeys that received ipilimumab every 3 weeks from the onset of organogenesis in the first trimester through parturition, developmental abnormalities were identified in the urogenital system of 2 infant monkeys exposed in utero to 30 mg/kg of ipilimumab (7.2 times the AUC in humans at the clinically recommended dose). One female infant monkey had unilateral renal agenesis of the left kidney and ureter, and 1 male infant monkey had an imperforate urethra with associated urinary obstruction and subcutaneous scrotal edema.
- Genetically engineered mice heterozygous for CTLA-4 (CTLA-4+/−), the target for ipilimumab, appeared healthy and gave birth to healthy CTLA-4+/− heterozygous offspring. Mated CTLA-4+/− heterozygous mice also produced offspring deficient in CTLA-4 (homozygous negative, CTLA-4−/−). The CTLA-4−/− homozygous negative offspring appeared healthy at birth, exhibited signs of multiorgan lymphoproliferative disease by 2 weeks of age, and all died by 3–4 weeks of age with massive lymphoproliferation and multiorgan tissue destruction.
Clinical Studies
The safety and efficacy of ipilimumab were investigated in a randomized (3:1:1), double-blind, double-dummy study (Study 1) that included 676 randomized patients with unresectable or metastatic melanoma previously treated with one or more of the following: aldesleukin, dacarbazine, temozolomide, fotemustine, or carboplatin. Of these 676 patients, 403 were randomized to receive ipilimumab at 3 mg/kg in combination with an investigational peptide vaccine with incomplete Freund’s adjuvant (gp100), 137 were randomized to receive ipilimumab at 3 mg/kg, and 136 were randomized to receive gp100 alone. The study enrolled only patients with HLA-A2*0201 genotype; this HLA genotype facilitates the immune presentation of the investigational peptide vaccine. The study excluded patients with active autoimmune disease or those receiving systemic immunosuppression for organ transplantation. ipilimumab/placebo was administered at 3 mg/kg as an intravenous infusion every 3 weeks for 4 doses. Gp100/placebo was administered at a dose of 2 mg peptide by deep subcutaneous injection every 3 weeks for 4 doses. Assessment of tumor response was conducted at weeks 12 and 24, and every 3 months thereafter. Patients with evidence of objective tumor response at 12 or 24 weeks had assessment for confirmation of durability of response at 16 or 28 weeks, respectively.
The major efficacy outcome measure was overall survival (OS) in the ipilimumab+gp100 arm compared to that in the gp100 arm. Secondary efficacy outcome measures were OS in the ipilimumab+gp100 arm compared to the ipilimumab arm, OS in the ipilimumab arm compared to the gp100 arm, best overall response rate (BORR) at week 24 between each of the study arms, and duration of response.
Of the randomized patients, 61%, 59%, and 54% in the ipilimumab+gp100, ipilimumab, and gp100 arms, respectively, were men. Twenty-nine percent were ≥65 years of age, the median age was 57 years, 71% had M1c stage, 12% had a history of previously treated brain metastasis, 98% had ECOG performance status of 0 and 1, 23% had received aldesleukin, and 38% had elevated LDH level. Sixty-one percent of patients randomized to either ipilimumab-containing arm received all 4 planned doses. The median duration of follow-up was 8.9 months.
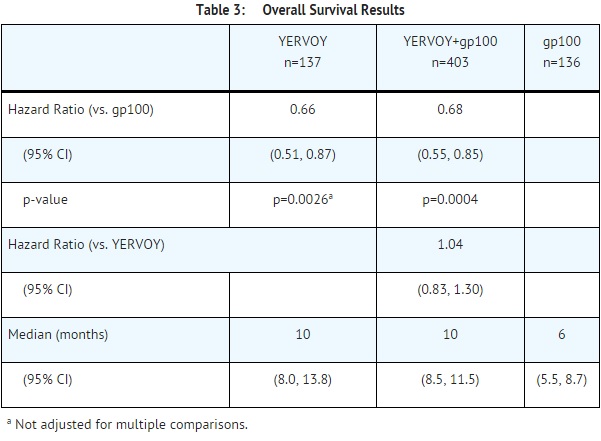
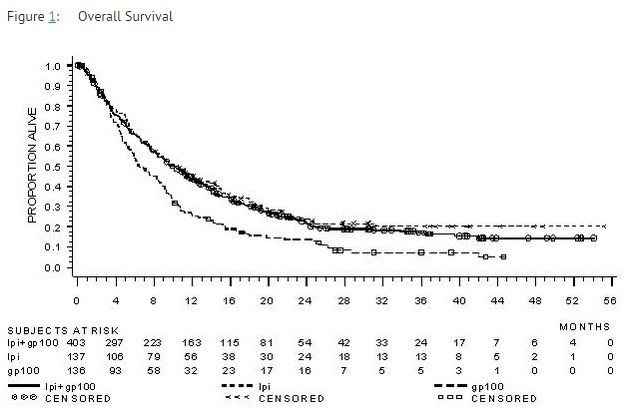
The best overall response rate (BORR) as assessed by the investigator was 5.7% (95% CI: 3.7%, 8.4%) in the ipilimumab+gp100 arm, 10.9% (95% CI: 6.3%, 17.4%) in the ipilimumab arm, and 1.5% (95% CI: 0.2%, 5.2%) in the gp100 arm. The median duration of response was 11.5 months in the ipilimumab+gp100 arm and has not been reached in the ipilimumab or gp100 arm.
How Supplied
50 mg vial (5 mg/mL), single-use vial
- NDC 0003-2327-11
200 mg vial (5 mg/mL), single-use vial
- NDC 0003-2328-22
Storage
Store ipilimumab under refrigeration at 2°C to 8°C (36°F to 46°F). Do not freeze. Protect vials from light.
Images
Drug Images
{{#ask: Page Name::Ipilimumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ipilimumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Inform patients of the potential risk of immune-mediated adverse reactions.
- Advise patients to read the ipilimumab Medication Guide before each ipilimumab infusion.
- Advise women that ipilimumab may cause fetal harm.
- Advise nursing mothers not to breastfeed while taking ipilimumab.
Precautions with Alcohol
Alcohol-ipilimumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Yervoy [1]
Look-Alike Drug Names
There is limited information regarding Ipilimumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Ipilimumab |Label Name=Ipilimumab 50 mg.jpg
}}
{{#subobject:
|Label Page=Ipilimumab |Label Name=Ipilimumab 200 mg.jpg
}}