Infliximab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: SERIOUS INFECTIONS and MALIGNANCY
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS: Patients treated with infliximab are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
infliximab should be discontinued if a patient develops a serious infection or sepsis. Reported infections include:
The risks and benefits of treatment with infliximab should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with infliximab, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. MALIGNANCY: Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including infliximab. Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers including infliximab. These cases have had a very aggressive disease course and have been fatal. All reported infliximab cases have occurred in patients with Crohn's disease or ulcerative colitis and the majority were in adolescent and young adult males. All of these patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with infliximab at or prior to diagnosis. |
Overview
Infliximab is a tumor necrosis factor blocker that is FDA approved for the treatment of crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash, abdominal pain, nausea, headache, pharyngitis, respiratory tract infection, fatigue, fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Crohn's Disease
- Dosing information
- The recommended dose of infliximab is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adults with moderately to severely active Crohn's disease or fistulizing Crohn's disease. For adult patients who respond and then lose their response, consideration may be given to treatment with 10 mg/kg. Patients who do not respond by Week 14 are unlikely to respond with continued dosing and consideration should be given to discontinue infliximab in these patients.
Ulcerative Colitis
- Dosing information
- The recommended dose of infliximab is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of adult patients with moderately to severely active ulcerative colitis.
Rheumatoid Arthritis
- Dosing Information
- The recommended dose of infliximab is 3 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 3 mg/kg every 8 weeks thereafter for the treatment of moderately to severely active rheumatoid arthritis. infliximab should be given in combination with methotrexate. For patients who have an incomplete response, consideration may be given to adjusting the dose up to 10 mg/kg or treating as often as every 4 weeks bearing in mind that risk of serious infections is increased at higher doses.
Ankylosing Spondylitis
- Dosing Information
- The recommended dose of infliximab is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 6 weeks thereafter for the treatment of active ankylosing spondylitis.
Psoriatic Arthritis
- Dosing Information
- The recommended dose of infliximab is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of psoriatic arthritis. infliximab can be used with or without methotrexate.
Plaque Psoriasis
- Dosing Information
- The recommended dose of infliximab is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks thereafter for the treatment of chronic severe (i.e., extensive and/or disabling) plaque psoriasis.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Infliximab in adult patients.
Non–Guideline-Supported Use
Adult Onset Still's Disease
- Dosing Information
Severe Refractory Hidradenitis Supurativa
- Dosing Information
- Administer IV infusions of 5 mg/kg until disease is controlled.[3]
Rheumathoid Arthritis Mnitherapy
- Dosing Information
- Administer IV infusions of 1 to 10 mg/kg.[4]
Synovitis
- Dosing Information
- Administer IV infusions of 3 mg/kg at weeks 0, 2, 6, followed by IV infusions every 8 weeks.[5]
Refractory Takayau Arteritis
- Dosing Information
- Administer IV infusions of 3-5 mg/kg every 4-8 weeks.[6]
Refractory Uveitis
- Dosing Information
- Administer IV infusion of 3-6 mg/kg, initially every 2, 4, and 6 weeks, followed by every 4-8 weeks according to treatment response.[7]
Refractory Wegener's Granulomatosis
- Dosing Information
- Administer IV infusions of 5 mg/kg on the first, fourteenth, and forty-second day, followed by infusions every 8 weeks.[8]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Crohn's Disease
- Dosing Information
- The recommended dose of infliximab for pediatric patients 6 years and older with moderately to severely active Crohn's disease is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks.
Ulcerative Colitis
- Dosing information
- The recommended dose of infliximab for pediatric patients 6 years and older with moderately to severely active ulcerative colitis is 5 mg/kg given as an intravenous induction regimen at 0, 2 and 6 weeks followed by a maintenance regimen of 5 mg/kg every 8 weeks.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Infliximab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Infliximab in pediatric patients.
Contraindications
- Moderate to severe heart failure.
- Hypersensitivity reaction to infliximab or to inactive components of the product or to any murine proteins.
Warnings
|
WARNING: SERIOUS INFECTIONS and MALIGNANCY
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS: Patients treated with infliximab are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
infliximab should be discontinued if a patient develops a serious infection or sepsis. Reported infections include:
The risks and benefits of treatment with infliximab should be carefully considered prior to initiating therapy in patients with chronic or recurrent infection. Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with infliximab, including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. MALIGNANCY: Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, including infliximab. Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers including infliximab. These cases have had a very aggressive disease course and have been fatal. All reported infliximab cases have occurred in patients with Crohn's disease or ulcerative colitis and the majority were in adolescent and young adult males. All of these patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with infliximab at or prior to diagnosis. |
Serious Infections
- Patients treated with infliximab are at increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
- Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, or parasitic organisms including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis,[] legionellosis, listeriosis, pneumocystosis and tuberculosis have been reported with TNF-blockers. Patients have frequently presented with disseminated rather than localized disease.
- Treatment with infliximab should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions and/or patients taking concomitant immunosuppressants such as corticosteroids or methrotexate may be at greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- With chronic or recurrent infection.
- Who have been exposed to tuberculosis.
- With a history of an opportunistic infection.
- Who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis.
- With underlying conditions that may predispose them to infection.
Tuberculosis
- Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving infliximab, including patients who have previously received treatment for latent or active tuberculosis. Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating infliximab and periodically during therapy.
- Treatment of latent tuberculosis infection prior to therapy with TNF blocking agents has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating infliximab, even for patients previously vaccinated with Bacille Calmette-Guerin (BCG).
- Anti-tuberculosis therapy should also be considered prior to initiation of infliximab in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
- Tuberculosis should be strongly considered in patients who develop a new infection during infliximab treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
Monitoring
- Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with infliximab, including the development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. Tests for latent tuberculosis infection may also be falsely negative while on therapy with infliximab.
- Infliximab should be discontinued if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with infliximab should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
Invasive Fungal Infections
For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric antifungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric antifungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and the risks of antifungal therapy.
Malignancies
Malignancies, some fatal, have been reported among children, adolescents and young adults who received treatment with TNF-blocking agents (initiation of therapy ≤ 18 years of age), including infliximab. Approximately half of these cases were lymphomas, including Hodgkin's lymphoma and non-Hodgkin's lymphoma. The other cases represented a variety of malignancies, including rare malignancies that are usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months (range 1 to 84 months) after the first dose of TNF blocker therapy. Most of the patients were receiving concomitant immunosuppressants. These cases were reported post-marketing and are derived from a variety of sources, including registries and spontaneous postmarketing reports.
Lymphomas
- In the controlled portions of clinical trials of all the TNF-blocking agents, more cases of lymphoma have been observed among patients receiving a TNF blocker compared with control patients. In the controlled and open-label portions of infliximab clinical trials, 5 patients developed lymphomas among 5707 patients treated with infliximab (median duration of follow-up 1.0 years) vs. 0 lymphomas in 1600 control patients (median duration of follow-up 0.4 years). In rheumatoid arthritis patients, 2 lymphomas were observed for a rate of 0.08 cases per 100 patient-years of follow-up, which is approximately three-fold higher than expected in the general population. In the combined clinical trial population for rheumatoid arthritis, Crohn's disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, 5 lymphomas were observed for a rate of 0.10 cases per 100 patient-years of follow-up, which is approximately four-fold higher than expected in the general population. Patients with Crohn's disease, rheumatoid arthritis or plaque psoriasis, particularly patients with highly active disease and/or chronic exposure to immunosuppressant therapies, may be at a higher risk (up to several fold) than the general population for the development of lymphoma, even in the absence of TNF-blocking therapy. Cases of acute and chronic leukemia have been reported with postmarketing TNF-blocker use in rheumatoid arthritis and other indications. Even in the absence of TNF blocker therapy, patients with rheumatoid arthritis may be at a higher risk (approximately 2-fold) than the general population for the development of leukemia.
- Hepatosplenic T-cell lymphoma (HSTCL)
- Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers including infliximab. These cases have had a very aggressive disease course and have been fatal. All reported infliximab cases have occurred in patients with Crohn's disease or ulcerative colitis and the majority were in adolescent and young adult males. All of these patients had received treatment with the immunosuppressants azathioprine or 6-mercaptopurine concomitantly with infliximab at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to infliximab or infliximab in combination with these other immunosuppressants. When treating patients with inflammatory bowel disease, particularly in adolescents and young adults, consideration of whether to use infliximab alone or in combination with other immunosuppressants should take into account a possibility that there is a higher risk of HSTCL with combination therapy versus an observed increased risk of immunogenicity and hypersensitivity reactions with infliximab monotherapy from the clinical trial data.
Skin cancer
Melanoma and Merkel cell carcinoma have been reported in patients treated with TNF blocker therapy, including infliximab. Periodic skin examination is recommended for all patients, particularly those with risk factors for skin cancer.
Other Malignancies
- In the controlled portions of clinical trials of some TNF-blocking agents including infliximab, more malignancies (excluding lymphoma and nonmelanoma skin cancer [NMSC]) have been observed in patients receiving those TNF-blockers compared with control patients. During the controlled portions of infliximab trials in patients with moderately to severely active rheumatoid arthritis, Crohn's disease, psoriatic arthritis, ankylosing spondylitis, ulcerative colitis, and plaque psoriasis, 14 patients were diagnosed with malignancies (excluding lymphoma and NMSC) among 4019 infliximab-treated patients vs. 1 among 1597 control patients (at a rate of 0.52/100 patient-years among infliximab-treated patients vs. a rate of 0.11/100 patient-years among control patients), with median duration of follow-up 0.5 years for infliximab-treated patients and 0.4 years for control patients. Of these, the most common malignancies were breast cancer, colorectal cancer, and melanoma. The rate of malignancies among infliximab-treated patients was similar to that expected in the general population whereas the rate in control patients was lower than expected.
- In a clinical trial exploring the use of infliximab in patients with moderate to severe chronic obstructive pulmonary disease (COPD), more malignancies, the majority of lung or head and neck origin, were reported in infliximab-treated patients compared with control patients. All patients had a history of heavy smoking. Prescribers should exercise caution when considering the use of infliximab in patients with moderate to severe COPD.
- Psoriasis patients should be monitored for nonmelanoma skin cancers (NMSCs), particularly those patients who have had prior prolonged phototherapy treatment. In the maintenance portion of clinical trials for infliximab, NMSCs were more common in patients with previous phototherapy.
- The potential role of TNF-blocking therapy in the development of malignancies is not known. Rates in clinical trials for infliximab cannot be compared to rates in clinical trials of other TNF-blockers and may not predict rates observed in a broader patient population. Caution should be exercised in considering infliximab treatment in patients with a history of malignancy or in continuing treatment in patients who develop malignancy while receiving infliximab.
Hepatitis B Virus Reactivation
- Use of TNF blockers, including infliximab, has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of these reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation. Patients should be tested for HBV infection before initiating TNF blocker therapy, including infliximab. For patients who test positive for hepatitis B surface antigen, consultation with a physician with expertise in the treatment of hepatitis B is recommended. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF blocker therapy to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with TNF blockers should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy. In patients who develop HBV reactivation, TNF blockers should be stopped and antiviral therapy with appropriate supportive treatment should be initiated. The safety of resuming TNF blocker therapy after HBV reactivation is controlled is not known. Therefore, prescribers should exercise caution when considering resumption of TNF blocker therapy in this situation and monitor patients closely.
Hepatotoxicity
- Severe hepatic reactions, including acute liver failure, jaundice, hepatitis and cholestasis, have been reported rarely in postmarketing data in patients receiving infliximab. Autoimmune hepatitis has been diagnosed in some of these cases. Severe hepatic reactions occurred between 2 weeks to more than 1 year after initiation of infliximab; elevations in hepatic aminotransferase levels were not noted prior to discovery of the liver injury in many of these cases. Some of these cases were fatal or necessitated liver transplantation. Patients with symptoms or signs of liver dysfunction should be evaluated for evidence of liver injury. If jaundice and/or marked liver enzyme elevations (e.g., ≥5 times the upper limit of normal) develop, infliximab should be discontinued, and a thorough investigation of the abnormality should be undertaken. In clinical trials, mild or moderate elevations of ALT and AST have been observed in patients receiving infliximab without progression to severe hepatic injury.
Patients with Heart Failure
- Infliximab has been associated with adverse outcomes in patients with heart failure, and should be used in patients with heart failure only after consideration of other treatment options. The results of a randomized study evaluating the use of infliximab in patients with heart failure (NYHA Functional Class III/IV) suggested higher mortality in patients who received 10 mg/kg infliximab, and higher rates of cardiovascular adverse events at doses of 5 mg/kg and 10 mg/kg. There have been post-marketing reports of worsening heart failure, with and without identifiable precipitating factors, in patients taking infliximab. There have also been rare post-marketing reports of new onset heart failure, including heart failure in patients without known pre-existing cardiovascular disease. Some of these patients have been under 50 years of age. If a decision is made to administer infliximab to patients with heart failure, they should be closely monitored during therapy, and infliximab should be discontinued if new or worsening symptoms of heart failure appear.
Hematologic Reactions
- Cases of leukopenia, neutropenia, thrombocytopenia, and pancytopenia, some with a fatal outcome, have been reported in patients receiving infliximab. The causal relationship to infliximab therapy remains unclear. Although no high-risk group(s) has been identified, caution should be exercised in patients being treated with infliximab who have ongoing or a history of significant hematologic abnormalities. All patients should be advised to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever) while on infliximab. Discontinuation of infliximab therapy should be considered in patients who develop significant hematologic abnormalities.
Hypersensitivity
- Infliximab has been associated with hypersensitivity reactions that vary in their time of onset and required hospitalization in some cases. Most hypersensitivity reactions, which include urticaria, dyspnea, and/or hypotension, have occurred during or within 2 hours of infliximab infusion.
- However, in some cases, serum sickness-like reactions have been observed in patients after initial infliximab therapy (i.e., as early as after the second dose), and when infliximab therapy was reinstituted following an extended period without infliximab treatment. Symptoms associated with these reactions include fever, rash, headache, sore throat, myalgias, polyarthralgias, hand and facial edema and/or dysphagia. These reactions were associated with a marked increase in antibodies to infliximab, loss of detectable serum concentrations of infliximab, and possible loss of drug efficacy.
- Infliximab should be discontinued for severe hypersensitivity reactions. Medications for the treatment of hypersensitivity reactions (e.g., acetaminophen, antihistamines, corticosteroids and/or epinephrine) should be available for immediate use in the event of a reaction.
- In rheumatoid arthritis, Crohn's disease and psoriasis clinical trials, re-administration of infliximab after a period of no treatment resulted in a higher incidence of infusion reactions relative to regular maintenance treatment. In general, the benefit-risk of re-administration of infliximab after a period of no-treatment, especially as a re-induction regimen given at weeks 0, 2 and 6, should be carefully considered. In the case where infliximab maintenance therapy for psoriasis is interrupted, infliximab should be reinitiated as a single dose followed by maintenance therapy.
Neurologic Reactions
- Infliximab and other agents that inhibit TNF have been associated in rare cases with CNS manifestation of systemic vasculitis, seizure and new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disorders, including multiple sclerosis and optic neuritis, and peripheral demyelinating disorders, including Guillain-Barré syndrome. Prescribers should exercise caution in considering the use of infliximab in patients with these neurologic disorders and should consider discontinuation of infliximab if these disorders develop.
Use with Anakinra
- Serious infections and neutropenia were seen in clinical studies with concurrent use of anakinra and another TNFα-blocking agent, etanercept, with no added clinical benefit compared to etanercept alone. Because of the nature of the adverse reactions seen with the combination of etanercept and anakinra therapy, similar toxicities may also result from the combination of anakinra and other TNFα-blocking agents. Therefore, the combination of infliximab and anakinra is not recommended.
Use with Abatacept
- In clinical studies, concurrent administration of TNF-blocking agents and abatacept have been associated with an increased risk of infections including serious infections compared with TNF-blocking agents alone, without increased clinical benefit. Therefore, the combination of infliximab and abatacept is not recommended.
Concurrent Administration with other Biological Therapeutics
- There is insufficient information regarding the concomitant use of infliximab with other biological therapeutics used to treat the same conditions as infliximab. The concomitant use of infliximab with these biologics is not recommended because of the possibility of an increased risk of infection.
Switching between Biological Disease-Modifying Antirheumatic Drugs (DMARDs)
- Care should be taken when switching from one biologic to another, since overlapping biological activity may further increase the risk of infection.
Autoimmunity
- Treatment with infliximab may result in the formation of autoantibodies and, rarely, in the development of a lupus-like syndrome. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with infliximab, treatment should be discontinued.
Live Vaccines/Therapeutic Infectious Agents
- In patients receiving anti-TNF therapy, limited data are available on the response to vaccination with live vaccines or on the secondary transmission of infection by live vaccines. Use of live vaccines could result in clinical infections, including disseminated infections. It is recommended that live vaccines not be given concurrently with infliximab. Caution is advised in the administration of live vaccines to infants born to female patients treated with infliximab during pregnancy since infliximab is known to cross the placenta and has been detected up to 6 months in the serum of infants born to female patients treated with infliximab during pregnancy.
- Other uses of therapeutic infectious agents such as live attenuated bacteria (e.g., BCG bladder instillation for the treatment of cancer) could result in clinical infections, including disseminated infections. It is recommended that therapeutic infectious agents not be given concurrently with infliximab.
- It is recommended that all pediatric patients be brought up to date with all vaccinations prior to initiating infliximab therapy. The interval between vaccination and initiation of infliximab therapy should be in accordance with current vaccination guidelines.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not predict the rates observed in broader patient populations in clinical practice.
Adverse Reactions in Adults
- The data described herein reflect exposure to infliximab in 4779 adult patients (1304 patients with rheumatoid arthritis, 1106 patients with Crohn's disease, 202 with ankylosing spondylitis, 293 with psoriatic arthritis, 484 with ulcerative colitis, 1373 with plaque psoriasis, and 17 patients with other conditions), including 2625 patients exposed beyond 30 weeks and 374 exposed beyond 1 year. One of the most-common reasons for discontinuation of treatment was infusion-related reactions (e.g., dyspnea, flushing, headache and rash).
- An infusion reaction was defined in clinical trials as any adverse event occurring during an infusion or within 1 hour after an infusion. In phase 3 clinical studies, 18% of infliximab-treated patients experienced an infusion reaction compared to 5% of placebo-treated patients. Of infliximab-treated patients who had an infusion reaction during the induction period, 27% experienced an infusion reaction during the maintenance period. Of patients who did not have an infusion reaction during the induction period, 9% experienced an infusion reaction during the maintenance period.
- Among all infliximab infusions, 3% were accompanied by nonspecific symptoms such as fever or chills, 1% were accompanied by cardiopulmonary reactions (primarily chest pain, hypotension, hypertension or dyspnea), and <1% were accompanied by pruritus, urticaria, or the combined symptoms of pruritus/urticaria and cardiopulmonary reactions. Serious infusion reactions occurred in <1% of patients and included anaphylaxis, convulsions, erythematous rash and hypotension. Approximately 3% of patients discontinued infliximab because of infusion reactions, and all patients recovered with treatment and/or discontinuation of the infusion. infliximab infusions beyond the initial infusion were not associated with a higher incidence of reactions. The infusion reaction rates remained stable in psoriasis through 1 year in psoriasis Study I. In psoriasis Study II, the rates were variable over time and somewhat higher following the final infusion than after the initial infusion. Across the 3 psoriasis studies, the percent of total infusions resulting in infusion reactions (i.e., an adverse event occurring within 1 hour) was 7% in the 3 mg/kg group, 4% in the 5 mg/kg group, and 1% in the placebo group.
- Patients who became positive for antibodies to infliximab were more likely (approximately two- to three-fold) to have an infusion reaction than were those who were negative. Use of concomitant immunosuppressant agents appeared to reduce the frequency of both antibodies to infliximab and infusion reactions.
Infusion reactions following re-administration
- In a clinical trial of patients with moderate to severe psoriasis designed to assess the efficacy of long-term maintenance therapy versus re-treatment with an induction regimen of infliximab following disease flare, 4% (8/219) of patients in the re-treatment therapy arm experienced serious infusion reactions versus < 1% (1/222) in the maintenance therapy arm. Patients enrolled in this trial did not receive any concomitant immunosuppressant therapy. In this study, the majority of serious infusion reactions occurred during the second infusion at Week 2. Symptoms included, but were not limited to, dyspnea, urticaria, facial edema, and hypotension. In all cases, infliximab treatment was discontinued and/or other treatment instituted with complete resolution of signs and symptoms.
Delayed Reactions/Reactions Following Re-administration
In psoriasis studies, approximately 1% of infliximab-treated patients experienced a possible delayed hypersensitivity reaction, generally reported as serum sickness or a combination of arthralgia and/or myalgia with fever and/or rash. These reactions generally occurred within 2 weeks after repeat infusion.
Infections
- In infliximab clinical studies, treated infections were reported in 36% of infliximab-treated patients (average of 51 weeks of follow-up) and in 25% of placebo-treated patients (average of 37 weeks of follow-up). The infections most frequently reported were respiratory tract infections (including sinusitis, pharyngitis, and bronchitis) and urinary tract infections. Among infliximab-treated patients, serious infections included pneumonia, cellulitis, abscess, skin ulceration, sepsis, and bacterial infection. In clinical trials, 7 opportunistic infections were reported; 2 cases each of coccidioidomycosis (1 case was fatal) and histoplasmosis (1 case was fatal), and 1 case each of pneumocystosis, nocardiosis and cytomegalovirus. Tuberculosis was reported in 14 patients, 4 of whom died due to miliary tuberculosis. Other cases of tuberculosis, including disseminated tuberculosis, also have been reported post-marketing. Most of these cases of tuberculosis occurred within the first 2 months after initiation of therapy with infliximab and may reflect recrudescence of latent disease. In the 1-year placebo-controlled studies RA I and RA II, 5.3% of patients receiving infliximab every 8 weeks with MTX developed serious infections as compared to 3.4% of placebo patients receiving MTX. Of 924 patients receiving infliximab, 1.7% developed pneumonia and 0.4% developed TB, when compared to 0.3% and 0.0% in the placebo arm respectively. In a shorter (22-week) placebo-controlled study of 1082 RA patients randomized to receive placebo, 3 mg/kg or 10 mg/kg infliximab infusions at 0, 2, and 6 weeks, followed by every 8 weeks with MTX, serious infections were more frequent in the 10 mg/kg infliximab group (5.3%) than the 3 mg/kg or placebo groups (1.7% in both). During the 54-week Crohn's II Study, 15% of patients with fistulizing Crohn's disease developed a new fistula-related abscess.
- In infliximab clinical studies in patients with ulcerative colitis, infections treated with antimicrobials were reported in 27% of infliximab-treated patients (average of 41 weeks of follow-up) and in 18% of placebo-treated patients (average 32 weeks of follow-up). The types of infections, including serious infections, reported in patients with ulcerative colitis were similar to those reported in other clinical studies.
- The onset of serious infections may be preceded by constitutional symptoms such as fever, chills, weight loss, and fatigue. The majority of serious infections, however, may also be preceded by signs or symptoms localized to the site of the infection.
Autoantibodies/Lupus-like Syndrome
- Approximately half of infliximab-treated patients in clinical trials who were antinuclear antibody (ANA) negative at baseline developed a positive ANA during the trial compared with approximately one-fifth of placebo-treated patients. Anti-dsDNA antibodies were newly detected in approximately one-fifth of infliximab-treated patients compared with 0% of placebo-treated patients. Reports of lupus and lupus-like syndromes, however, remain uncommon.
Malignancies
- In controlled trials, more infliximab-treated patients developed malignancies than placebo-treated patients.
- In a randomized controlled clinical trial exploring the use of infliximab in patients with moderate to severe COPD who were either current smokers or ex-smokers, 157 patients were treated with infliximab at doses similar to those used in rheumatoid arthritis and Crohn's disease. Of these infliximab-treated patients, 9 developed a malignancy, including 1 lymphoma, for a rate of 7.67 cases per 100 patient-years of follow-up (median duration of follow-up 0.8 years; 95% CI 3.51 – 14.56). There was 1 reported malignancy among 77 control patients for a rate of 1.63 cases per 100 patient-years of follow-up (median duration of follow-up 0.8 years; 95% CI 0.04 – 9.10). The majority of the malignancies developed in the lung or head and neck.
Patients with Heart Failure
- In a randomized study evaluating infliximab in moderate to severe heart failure (NYHA Class III/IV; left ventricular ejection fraction ≤35%), 150 patients were randomized to receive treatment with 3 infusions of infliximab 10 mg/kg, 5 mg/kg, or placebo, at 0, 2, and 6 weeks. Higher incidences of mortality and hospitalization due to worsening heart failure were observed in patients receiving the 10 mg/kg infliximab dose. At 1 year, 8 patients in the 10 mg/kg infliximab group had died compared with 4 deaths each in the 5 mg/kg infliximab and the placebo groups. There were trends toward increased dyspnea, hypotension, angina, and dizziness in both the 10 mg/kg and 5 mg/kg infliximab treatment groups, versus placebo. infliximab has not been studied in patients with mild heart failure (NYHA Class I/II).
Immunogenicity
- Treatment with infliximab can be associated with the development of antibodies to infliximab. The assay used to measure anti-infliximab antibodies in patient samples is subject to interference by serum infliximab, possibly resulting in an underestimation of the rate of patient antibody formation. The incidence of antibodies to infliximab in patients given a 3-dose induction regimen followed by maintenance dosing was approximately 10% as assessed through 1 to 2 years of infliximab treatment. A higher incidence of antibodies to infliximab was observed in Crohn's disease patients receiving infliximab after drug-free intervals >16 weeks. In a study of psoriatic arthritis in which 191 patients received 5 mg/kg with or without MTX, antibodies to infliximab occurred in 15% of patients. The majority of antibody-positive patients had low titers. Patients who were antibody-positive were more likely to have higher rates of clearance, reduced efficacy and to experience an infusion reaction than were patients who were antibody negative. Antibody development was lower among rheumatoid arthritis and Crohn's disease patients receiving immunosuppressant therapies such as 6-MP/AZA or MTX.
- In the psoriasis Study II, which included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 36% of patients treated with 5 mg/kg every 8 weeks for 1 year, and in 51% of patients treated with 3 mg/kg every 8 weeks for 1 year. In the psoriasis Study III, which also included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 20% of patients treated with 5 mg/kg induction (weeks 0, 2 and 6), and in 27% of patients treated with 3 mg/kg induction. Despite the increase in antibody formation, the infusion reaction rates in Studies I and II in patients treated with 5 mg/kg induction followed by every 8 week maintenance for 1 year and in Study III in patients treated with 5 mg/kg induction (14.1%–23.0%) and serious infusion reaction rates (<1%) were similar to those observed in other study populations. The clinical significance of apparent increased immunogenicity on efficacy and infusion reactions in psoriasis patients as compared to patients with other diseases treated with infliximab over the long term is not known.
- The data reflect the percentage of patients whose test results were positive for antibodies to infliximab in an ELISA assay, and they are highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody positivity in an assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medication, and underlying disease. For these reasons, comparison of the incidence of antibodies to infliximab with the incidence of antibodies to other products may be misleading.
Hepatotoxicity
Severe liver injury, including acute liver failure and autoimmune hepatitis, has been reported rarely in patients receiving infliximab. Reactivation of hepatitis B virus has occurred in patients receiving TNF-blocking agents, including infliximab, who are chronic carriers of this virus. In clinical trials in rheumatoid arthritis, Crohn's disease, ulcerative colitis, ankylosing spondylitis, plaque psoriasis, and psoriatic arthritis, elevations of aminotransferases were observed (ALT more common than AST) in a greater proportion of patients receiving infliximab than in controls the table below, both when infliximab was given as monotherapy and when it was used in combination with other immunosuppressive agents. In general, patients who developed ALT and AST elevations were asymptomatic, and the abnormalities decreased or resolved with either continuation or discontinuation of infliximab, or modification of concomitant medications.
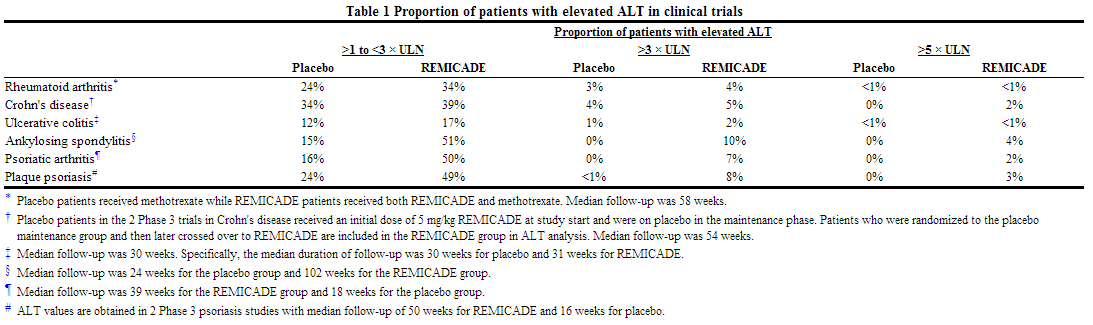
Adverse Reactions in Psoriasis Studies
- During the placebo-controlled portion across the 3 clinical trials up to week 16, the proportion of patients who experienced at least 1 serious adverse reaction (SAE; defined as resulting in death, life threatening, requires hospitalization, or persistent or significant disability/incapacity) was 0.5% in the 3 mg/kg infliximab group, 1.9% in the placebo group, and 1.6% in the 5 mg/kg infliximab group.
- Among patients in the 2 Phase 3 studies, 12.4% of patients receiving infliximab 5 mg/kg every 8 weeks through 1 year of maintenance treatment experienced at least 1 SAE in Study I. In Study II, 4.1% and 4.7% of patients receiving infliximab 3 mg/kg and 5 mg/kg every 8 weeks, respectively, through 1 year of maintenance treatment experienced at least 1 SAE.
- One death due to bacterial sepsis occurred 25 days after the second infusion of 5 mg/kg infliximab. Serious infections included sepsis, and abscesses. In Study I, 2.7% of patients receiving infliximab 5 mg/kg every 8 weeks through 1 year of maintenance treatment experienced at least 1 serious infection. In Study II, 1.0% and 1.3% of patients receiving infliximab 3 mg/kg and 5 mg/kg, respectively, through 1 year of treatment experienced at least 1 serious infection. The most common serious infection (requiring hospitalization) was abscess (skin, throat, and peri-rectal) reported by 5 (0.7%) patients in the 5 mg/kg infliximab group. Two active cases of tuberculosis were reported: 6 weeks and 34 weeks after starting infliximab.
- In the placebo-controlled portion of the psoriasis studies, 7 of 1123 patients who received infliximab at any dose were diagnosed with at least one NMSC compared to 0 of 334 patients who received placebo.
- In the psoriasis studies, 1% (15/1373) of patients experienced serum sickness or a combination of arthralgia and/or myalgia with fever, and/or rash, usually early in the treatment course. Of these patients, 6 required hospitalization due to fever, severe myalgia, arthralgia, swollen joints, and immobility.
Other Adverse Reactions
- Safety data are available from 4779 infliximab-treated adult patients, including 1304 with rheumatoid arthritis, 1106 with Crohn's disease, 484 with ulcerative colitis, 202 with ankylosing spondylitis, 293 with psoriatic arthritis, 1373 with plaque psoriasis and 17 with other conditions. Adverse reactions reported in ≥5% of all patients with rheumatoid arthritis receiving 4 or more infusions are in Table 2. The types and frequencies of adverse reactions observed were similar in infliximab-treated rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, plaque psoriasis and Crohn's disease patients except for abdominal pain, which occurred in 26% of infliximab-treated patients with Crohn's disease. In the Crohn's disease studies, there were insufficient numbers and duration of follow-up for patients who never received infliximab to provide meaningful comparisons.
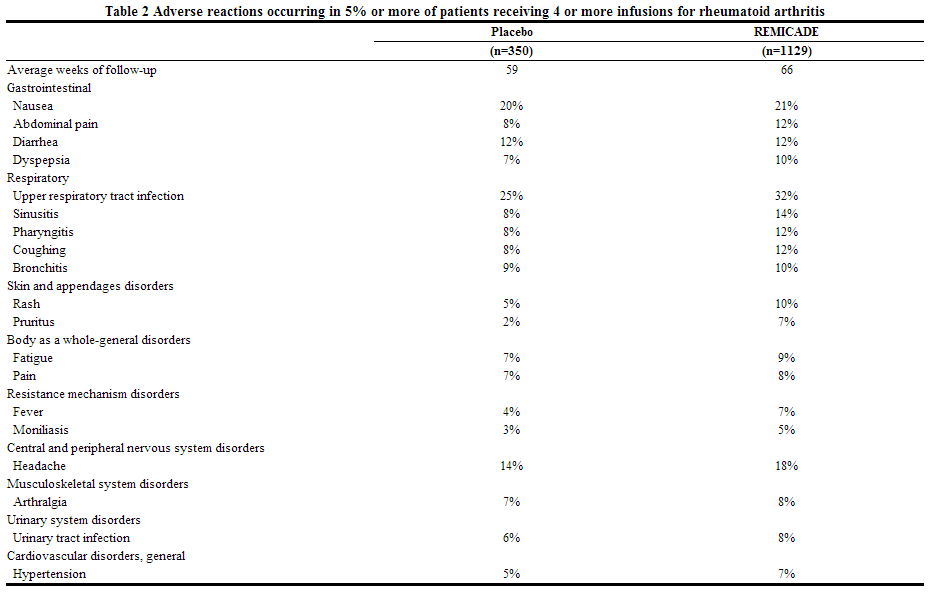
The most common serious adverse reactions observed in clinical trials were infections [see Adverse Reactions (6.1)]. Other serious, medically relevant adverse reactions ≥0.2% or clinically significant adverse reactions by body system were as follows:
- Body as a whole: Allergic reaction, edema
- Blood: Pancytopenia
- Cardiovascular: Hypotension
- Gastrointestinal: Constipation, intestinal obstruction
- Central and Peripheral Nervous: Dizziness
- Heart Rate and Rhythm: Bradycardia
- Liver and Biliary: Hepatitis
- Metabolic and Nutritional: Dehydration
- Platelet, Bleeding and Clotting: Thrombocytopenia
- Neoplasms: Lymphoma
- Red Blood Cell: Anemia, hemolytic anemia
- Resistance Mechanism: Cellulitis, sepsis, serum sickness, sarcoidosis
- Respiratory: Lower respiratory tract infection (including pneumonia), pleurisy, pulmonary edema
- Skin and Appendages: Increased sweating
- Vascular (Extracardiac): Thrombophlebitis
- White Cell and Reticuloendothelial: Leukopenia, lymphadenopathy
Adverse Reactions in Pediatric Patients
Pediatric Crohn's Disease
- There were some differences in the adverse reactions observed in the pediatric patients receiving infliximab compared to those observed in adults with Crohn's disease. These differences are discussed in the following paragraphs.
- The following adverse reactions were reported more commonly in 103 randomized pediatric Crohn's disease patients administered 5 mg/kg infliximab through 54 weeks than in 385 adult Crohn's disease patients receiving a similar treatment regimen: anemia (11%), leukopenia (9%), flushing (9%), viral infection (8%), neutropenia (7%), bone fracture (7%), bacterial infection (6%), and respiratory tract allergic reaction (6%).
- Infections were reported in 56% of randomized pediatric patients in Study Peds Crohn's and in 50% of adult patients in Study Crohn's I. In Study Peds Crohn's, infections were reported more frequently for patients who received every 8-week as opposed to every 12-week infusions (74% and 38%, respectively), while serious infections were reported for 3 patients in the every 8-week and 4 patients in the every 12-week maintenance treatment group. The most commonly reported infections were upper respiratory tract infection and pharyngitis, and the most commonly reported serious infection was abscess. Pneumonia was reported for 3 patients, (2 in the every 8-week and 1 in the every 12-week maintenance treatment groups). Herpes zoster was reported for 2 patients in the every 8-week maintenance treatment group.
- In Study Peds Crohn's, 18% of randomized patients experienced 1 or more infusion reactions, with no notable difference between treatment groups. Of the 112 patients in Study Peds Crohn's, there were no serious infusion reactions, and 2 patients had non-serious anaphylactoid reactions.
- In Study Peds Crohn's, in which all patients received stable doses of 6-MP, AZA, or MTX, excluding inconclusive samples, 3 of 24 patients had antibodies to infliximab. Although 105 patients were tested for antibodies to infliximab, 81 patients were classified as inconclusive because they could not be ruled as negative due to assay interference by the presence of infliximab in the sample.
- Elevations of ALT up to 3 times the upper limit of normal (ULN) were seen in 18% of pediatric patients in Crohn's disease clinical trials; 4% had ALT elevations ≥3 × ULN, and 1% had elevations ≥5 × ULN. (Median follow-up was 53 weeks.)
Pediatric Ulcerative Colitis
- Overall, the adverse reactions reported in the pediatric ulcerative colitis trial and adult ulcerative colitis (Study UC I and Study UC II) studies were generally consistent. In a pediatric UC trial, the most common adverse reactions were upper respiratory tract infection, pharyngitis, abdominal pain, fever, and headache.
- Infections were reported in 31 (52%) of 60 treated patients in the pediatric UC trial and 22 (37%) required oral or parenteral antimicrobial treatment. The proportion of patients with infections in the pediatric UC trial was similar to that in the pediatric Crohn's disease study (Study Peds Crohn's) but higher than the proportion in the adults' ulcerative colitis studies (Study UC I and Study UC II). The overall incidence of infections in the pediatric UC trial was 13/22 (59%) in the every 8 week maintenance treatment group. Upper respiratory tract infection (7/60 [12%]) and pharyngitis (5/60 [8%]) were the most frequently reported respiratory system infections. Serious infections were reported in 12% (7/60) of all treated patients.
- In the pediatric UC trial, excluding inconclusive samples, 4 of 19 patients had antibodies to infliximab. Although 52 patients were tested, 33 patients were classified as inconclusive because they could not be ruled as negative due to assay interference by the presence of infliximab in the sample.
- Elevations of ALT up to 3 times the upper limit of normal (ULN) were seen in 17% (10/60) of pediatric patients in the pediatric UC trial; 7% (4/60) had ALT elevations ≥3 × ULN, and 2% (1/60) had elevations ≥5 × ULN (median follow-up was 49 weeks).
- Overall, 8 of 60 (13%) treated patients experienced one or more infusion reactions, including 4 of 22 (18%) patients in the every 8-week treatment maintenance group. No serious infusion reactions were reported.
- In the pediatric UC trial, 45 patients were in the 12 to 17 year age group and 15 in the 6 to 11 year age group. The numbers of patients in each subgroup are too small to make any definitive conclusions about the effect of age on safety events. There were higher proportions of patients with serious adverse events (40% vs. 18%) and discontinuation due to adverse events (40% vs. 16%) in the younger age group than in the older age group. While the proportion of patients with infections was also higher in the younger age group (60% vs. 49%), for serious infections, the proportions were similar in the two age groups (13% in the 6 to 11 year age group vs. 11% in the 12 to 17 year age group). Overall proportions of adverse reactions, including infusion reactions, were similar between the 6 to 11 and 12 to 17 year age groups (13%).
Postmarketing Experience
Adverse reactions have been reported during post approval use of infliximab in adult and pediatric patients. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to infliximab exposure. The following adverse reactions, some with fatal outcome, have been reported during post-approval use of infliximab: neutropenia, interstitial lung disease (including pulmonary fibrosis/interstitial pneumonitis and very rare rapidly progressive disease), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, pericardial effusion, systemic and cutaneous vasculitis, erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis, peripheral demyelinating disorders (such as Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, and multifocal motor neuropathy), new onset and worsening psoriasis (all subtypes including pustular, primarily palmoplantar), transverse myelitis, and neuropathies (additional neurologic reactions have also been observed), acute liver failure, jaundice, hepatitis, and cholestasis, serious infections and malignancies, including melanoma and Merkel cell carcinoma.
- In post-marketing experience, cases of anaphylactic reactions, including laryngeal/pharyngeal edema and severe bronchospasm, and seizure have been associated with infliximab administration.
- Cases of myocardial ischemia and myocardial infarction and transient visual loss have also been rarely reported in association with infliximab during or within 2 hours of infusion.
Adverse Reactions in Pediatric Patients
- The following serious adverse reactions have been reported in the post-marketing experience in children: infections (some fatal) including opportunistic infections and tuberculosis, infusion reactions, and hypersensitivity reactions.
- Serious adverse reactions in the post-marketing experience with infliximab in the pediatric population have also included malignancies, including hepatosplenic T-cell lymphomas, transient hepatic enzyme abnormalities, lupus-like syndromes, and the development of autoantibodies.
Drug Interactions
Use with Anakinra or Abatacept
- An increased risk of serious infections was seen in clinical studies of other TNFα-blocking agents used in combination with anakinra or abatacept, with no added clinical benefit. Because of the nature of the adverse reactions seen with these combinations with TNF-blocker therapy, similar toxicities may also result from the combination of anakinra or abatacept with other TNFα-blocking agents. Therefore, the combination of infliximibe and anakinra or abatacept is not recommended.
Use with Tocilizumab
- The use of tocilizumab in combination with biological DMARDs such as TNF antagonists, including infliximibe, should be avoided because of the possibility of increased immunosuppression and increased risk of infection.
Use with Other Biological Therapeutics
- The combination of infliximibe with other biological therapeutics used to treat the same conditions as infliximibe is not recommended.
Methotrexate (MTX) and Other Concomitant Medications
- Specific drug interaction studies, including interactions with MTX, have not been conducted. The majority of patients in rheumatoid arthritis or Crohn's disease clinical studies received one or more concomitant medications. In rheumatoid arthritis, concomitant medications besides MTX were nonsteroidal anti-inflammatory agents (NSAIDs), folic acid, corticosteroids and/or narcotics. Concomitant Crohn's disease medications were antibiotics, antivirals, corticosteroids, 6-MP/AZA and aminosalicylates. In psoriatic arthritis clinical trials, concomitant medications included MTX in approximately half of the patients as well as NSAIDs, folic acid and corticosteroids. Concomitant MTX use may decrease the incidence of anti-infliximab antibody production and increase infliximab concentrations.
Immunosuppressants
- Patients with Crohn's disease who received immunosuppressants tended to experience fewer infusion reactions compared to patients on no immunosuppressants [see Adverse Reactions (6.1)]. Serum infliximab concentrations appeared to be unaffected by baseline use of medications for the treatment of Crohn's disease including corticosteroids, antibiotics (metronidazole or ciprofloxacin) and aminosalicylates.
Cytochrome P450 Substrates
- The formation of CYP450 enzymes may be suppressed by increased levels of cytokines (e.g., TNFα, IL-1, IL-6, IL-10, IFN) during chronic inflammation. Therefore, it is expected that for a molecule that antagonizes cytokine activity, such as infliximab, the formation of CYP450 enzymes could be normalized. Upon initiation or discontinuation of infliximibe in patients being treated with CYP450 substrates with a narrow therapeutic index, monitoring of the effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) is recommended and the individual dose of the drug product may be adjusted as needed.
Live Vaccines/Therapeutic Infectious Agents
- It is recommended that live vaccines not be given concurrently with infliximibe.
- It is recommended that therapeutic infectious agents not be given concurrently with infliximibe.
Use in Specific Populations
Pregnancy
- It is not known whether infliximab can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Infliximab should be given to a pregnant woman only if clearly needed. Because infliximab does not cross-react with TNFα in species other than humans and chimpanzees, animal reproduction studies have not been conducted with infliximab. No evidence of maternal toxicity, embryotoxicity or teratogenicity was observed in a developmental toxicity study conducted in mice using an analogous antibody that selectively inhibits the functional activity of mouse TNFα. Doses of 10 to 15 mg/kg in pharmacodynamic animal models with the anti-TNF analogous antibody produced maximal pharmacologic effectiveness. Doses up to 40 mg/kg were shown to produce no adverse effects in animal reproduction studies.
- As with other IgG antibodies, infliximab crosses the placenta and has been detected up to 6 months in the serum of infants born to female patients treated with infliximab during pregnancy. Consequently, these infants may be at increased risk of infection, and caution is advised in the administration of live vaccines to these infants
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Infliximab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Infliximab during labor and delivery.
Nursing Mothers
- It is not known whether infliximab is excreted in human milk or absorbed systemically after ingestion. Because many drugs and immunoglobulins are excreted in human milk, and because of the potential for adverse reactions in nursing infants from infliximab, women should not breast-feed their infants while taking infliximab. A decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of infliximibe have been established in pediatric patients 6 to 17 years of age for induction and maintenance treatment of Crohn's disease or ulcerative colitis. However, infliximibe has not been studied in children with Crohn's disease or ulcerative colitis <6 years of age.
Pediatric Crohn's Disease
- Infliximibe is indicated for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients with moderately to severely active Crohn's disease who have had an inadequate response to conventional therapy.
- Infliximibe has been studied only in combination with conventional immunosuppressive therapy in pediatric Crohn's disease. The longer term (greater than 1 year) safety and effectiveness of infliximibe in pediatric Crohn's disease patients have not been established in clinical trials.
Pediatric Ulcerative Colitis
- The safety and effectiveness of infliximibe for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients aged 6 years and older with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy are supported by evidence from adequate and well-controlled studies of infliximibe in adults. Additional safety and pharmacokinetic data were collected in 60 pediatric patients aged 6 years and older. The effectiveness of infliximibe in inducing and maintaining mucosal healing could not be established. Although 41 patients had a Mayo endoscopy subscore of 0 or 1 at the Week 8 endoscopy, the induction phase was open-label and lacked a control group. Only 9 patients had an optional endoscopy at Week 54.
- In the pediatric UC trial, approximately half of the patients were on concomitant immunomodulators (AZA, 6-MP, MTX) at study start. Due to the risk of HSTCL, a careful risk-benefit assessment should be made when infliximibe is used in combination with other immunosuppressants.
- The longer term (greater than 1 year) safety and effectiveness of infliximibe in pediatric ulcerative colitis patients have not been established in clinical trials.
Juvenile Rheumatoid Arthritis (JRA)
- The safety and efficacy of infliximibe in patients with juvenile rheumatoid arthritis (JRA) were evaluated in a multicenter, randomized, placebo-controlled, double-blind study for 14 weeks, followed by a double-blind, all-active treatment extension, for a maximum of 44 weeks. Patients with active JRA between the ages of 4 and 17 years who had been treated with MTX for at least 3 months were enrolled. Concurrent use of folic acid, oral corticosteroids (≤0.2 mg/kg/day of prednisone or equivalent), NSAIDs, and/or disease modifying antirheumatic drugs (DMARDs) was permitted.
- Doses of 3 mg/kg infliximibe or placebo were administered intravenously at Weeks 0, 2 and 6. Patients randomized to placebo crossed-over to receive 6 mg/kg infliximibe E at Weeks 14, 16, and 20, and then every 8 weeks through Week 44. Patients who completed the study continued to receive open-label treatment with infliximibe for up to 2 years in a companion extension study.
- The study failed to establish the efficacy of infliximibe in the treatment of JRA. Key observations in the study included a high placebo response rate and a higher rate of immunogenicity than what has been observed in adults. Additionally, a higher rate of clearance of infliximab was observed than had been observed in adults.
- A total of 60 patients with JRA were treated with doses of 3 mg/kg and 57 patients were treated with doses of 6 mg/kg. The proportion of patients with infusion reactions who received 3 mg/kg infliximibe was 35% (21/60) over 52 weeks compared with 18% (10/57) in patients who received 6 mg/kg over 38 weeks. The most common infusion reactions reported were vomiting, fever, headache, and hypotension. In the 3 mg/kg infliximibe group, 4 patients had a serious infusion reaction and 3 patients reported a possible anaphylactic reaction (2 of which were among the serious infusion reactions). In the 6 mg/kg infliximibe group, 2 patients had a serious infusion reaction, 1 of whom had a possible anaphylactic reaction. Two of the 6 patients who experienced serious infusion reactions received infliximibe by rapid infusion (duration of less than 2 hours). Antibodies to infliximab developed in 38% (20/53) of patients who received 3 mg/kg infliximibe compared with 12% (6/49) of patients who received 6 mg/kg.
- A total of 68% (41/60) of patients who received 3 mg/kg infliximibe in combination with MTX experienced an infection over 52 weeks compared with 65% (37/57) of patients who received 6 mg/kg infliximibe in combination with MTX over 38 weeks. The most commonly reported infections were upper respiratory tract infection and pharyngitis, and the most commonly reported serious infection was pneumonia. Other notable infections included primary varicella infection in 1 patient and herpes zoster in 1 patient.
Geriatic Use
In rheumatoid arthritis and plaque psoriasis clinical trials, no overall differences were observed in effectiveness or safety in 181 patients with rheumatoid arthritis and 75 patients with plaque psoriasis, aged 65 or older who received infliximibe , compared to younger patients - although the incidence of serious adverse reactions in patients aged 65 or older was higher in both infliximibe and control groups compared to younger patients. In Crohn's disease, ulcerative colitis, ankylosing spondylitis and psoriatic arthritis studies, there were insufficient numbers of patients aged 65 and over to determine whether they respond differently from patients aged 18 to 65. There is a greater incidence of infections in the elderly population in general. The incidence of serious infections in infliximibe-treated patients 65 years and older was greater than in those under 65 years of age; therefore caution should be used in treating the elderl
Gender
There is no FDA guidance on the use of Infliximab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Infliximab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Infliximab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Infliximab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Infliximab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Infliximab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
- Infliximab is intended for use under the guidance and supervision of a physician. The reconstituted infusion solution should be prepared by a trained medical professional using aseptic technique by the following procedure:
- Calculate the dose, total volume of reconstituted infliximab solution required and the number of infliximab vials needed. Each infliximab vial contains 100 mg of the infliximab antibody.
- Reconstitute each infliximab vial with 10 mL of Sterile Water for Injection, USP, using a syringe equipped with a 21-gauge or smaller needle as follows: Remove the flip-top from the vial and wipe the top with an alcohol swab. Insert the syringe needle into the vial through the center of the rubber stopper and direct the stream of Sterile Water for Injection, USP, to the glass wall of the vial. Gently swirl the solution by rotating the vial to dissolve the lyophilized powder. Avoid prolonged or vigorous agitation. Do not shake. Foaming of the solution on reconstitution is not unusual. Allow the reconstituted solution to stand for 5 minutes. The solution should be colorless to light yellow and opalescent, and the solution may develop a few translucent particles as infliximab is a protein. Do not use if the lyophilized cake has not fully dissolved or if opaque particles, discoloration, or other foreign particles are present.
- Dilute the total volume of the reconstituted infliximab solution dose to 250 mL with sterile 0.9% Sodium Chloride Injection, USP, by withdrawing a volume equal to the volume of reconstituted infliximab from the 0.9% Sodium Chloride Injection, USP, 250 mL bottle or bag. Slowly add the total volume of reconstituted infliximab solution to the 250 mL infusion bottle or bag. Gently mix. The resulting infusion concentration should range between 0.4 mg/mL and 4 mg/mL.
- The infliximab infusion should begin within 3 hours of reconstitution and dilution. The infusion must be administered over a period of not less than 2 hours and must use an infusion set with an in-line, sterile, non-pyrogenic, low-protein-binding filter (pore size of 1.2 µm or less). The vials do not contain antibacterial preservatives. Therefore, any unused portion of the infusion solution should not be stored for reuse.
- No physical biochemical compatibility studies have been conducted to evaluate the co-administration of infliximab with other agents. infliximab should not be infused concomitantly in the same intravenous line with other agents.
- Parenteral drug products should be inspected visually before and after reconstitution for particulate matter and discoloration prior to administration, whenever solution and container permit. If visibly opaque particles, discoloration or other foreign particulates are observed, the solution should not be used.
Monitoring
Prior to initiating infliximab and periodically during therapy, patients should be evaluated for active tuberculosis and tested for latent infection.
IV Compatibility
No physical biochemical compatibility studies have been conducted to evaluate the co-administration of infliximab with other agents. infliximab should not be infused concomitantly in the same intravenous line with other agents.
Overdosage
Single doses up to 20 mg/kg have been administered without any direct toxic effect. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects and appropriate symptomatic treatment instituted immediately.
Pharmacology
| |
Infliximab?
| |
| Therapeutic monoclonal antibody | |
| Source | xi/o |
| Target | TNF |
| Identifiers | |
| CAS number | |
| ATC code | L04 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 144190.3 g/mol |
| Pharmacokinetic data | |
| Bioavailability | 92% (IV, if 8% left in the syringe) |
| Metabolism | reticuloendothelial system |
| Half life | 9.5 days |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | IV |
Mechanism of Action
Infliximab neutralizes the biological activity of TNFα by binding with high affinity to the soluble and transmembrane forms of TNFα and inhibits binding of TNFα with its receptors. Infliximab does not neutralize TNFβ (lymphotoxin-α), a related cytokine that utilizes the same receptors as TNFα. Biological activities attributed to TNFα include: induction of pro-inflammatory cytokines such as interleukins (IL) 1 and 6, enhancement of leukocyte migration by increasing endothelial layer permeability and expression of adhesion molecules by endothelial cells and leukocytes, activation of neutrophil and eosinophil functional activity, induction of acute phase reactants and other liver proteins, as well as tissue degrading enzymes produced by synoviocytes and/or chondrocytes. Cells expressing transmembrane TNFα bound by infliximab can be lysed in vitro or in vivo. Infliximab inhibits the functional activity of TNFα in a wide variety of in vitro bioassays utilizing human fibroblasts, endothelial cells, neutrophils, B and T- lymphocytes and epithelial cells. The relationship of these biological response markers to the mechanism(s) by which infliximab exerts its clinical effects is unknown. Anti-TNFα antibodies reduce disease activity in the cotton-top tamarin colitis model, and decrease synovitis and joint erosions in a murine model of collagen-induced arthritis. Infliximab prevents disease in transgenic mice that develop polyarthritis as a result of constitutive expression of human TNFα, and when administered after disease onset, allows eroded joints to heal.
Structure
Infliximab, the active ingredient in infliximab, is a chimeric IgG1κ monoclonal antibody (composed of human constant and murine variable regions) specific for human tumor necrosis factor-alpha (TNFα). It has a molecular weight of approximately 149.1 kilodaltons. Infliximab is produced by a recombinant cell line cultured by continuous perfusion and is purified by a series of steps that includes measures to inactivate and remove viruses.
Infliximab is supplied as a sterile, white, lyophilized powder for intravenous infusion. Following reconstitution with 10 mL of Sterile Water for Injection, USP, the resulting pH is approximately 7.2. Each single-use vial contains 100 mg infliximab, 500 mg sucrose, 0.5 mg polysorbate 80, 2.2 mg monobasic sodium phosphate, monohydrate, and 6.1 mg dibasic sodium phosphate, dihydrate. No preservatives are present.

Pharmacodynamics
Elevated concentrations of TNFα have been found in involved tissues and fluids of patients with rheumatoid arthritis, Crohn's disease, ulcerative colitis, ankylosing spondylitis, psoriatic arthritis and plaque psoriasis. In rheumatoid arthritis, treatment with infliximab reduced infiltration of inflammatory cells into inflamed areas of the joint as well as expression of molecules mediating cellular adhesion [E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)], chemoattraction [IL-8 and monocyte chemotactic protein (MCP-1)] and tissue degradation [matrix metalloproteinase (MMP) 1 and 3]. In Crohn's disease, treatment with infliximab reduced infiltration of inflammatory cells and TNFα production in inflamed areas of the intestine, and reduced the proportion of mononuclear cells from the lamina propria able to express TNFα and interferon. After treatment with infliximab, patients with rheumatoid arthritis or Crohn's disease exhibited decreased levels of serum IL-6 and C-reactive protein (CRP) compared to baseline. Peripheral blood lymphocytes from infliximab-treated patients showed no significant decrease in number or in proliferative responses to in vitro mitogenic stimulation when compared to cells from untreated patients. In psoriatic arthritis, treatment with infliximab resulted in a reduction in the number of T-cells and blood vessels in the synovium and psoriatic skin lesions as well as a reduction of macrophages in the synovium. In plaque psoriasis, infliximab treatment may reduce the epidermal thickness and infiltration of inflammatory cells. The relationship between these pharmacodynamic activities and the mechanism(s) by which infliximab exerts its clinical effects is unknown.
Pharmacokinetics
In adults, single intravenous (IV) infusions of 3 mg/kg to 20 mg/kg showed a linear relationship between the dose administered and the maximum serum concentration. The volume of distribution at steady state was independent of dose and indicated that infliximab was distributed primarily within the vascular compartment. Pharmacokinetic results for single doses of 3 mg/kg to 10 mg/kg in rheumatoid arthritis, 5 mg/kg in Crohn's disease, and 3 mg/kg to 5 mg/kg in plaque psoriasis indicate that the median terminal half-life of infliximab is 7.7 to 9.5 days.
Following an initial dose of infliximab, repeated infusions at 2 and 6 weeks resulted in predictable concentration-time profiles following each treatment. No systemic accumulation of infliximab occurred upon continued repeated treatment with 3 mg/kg or 10 mg/kg at 4- or 8-week intervals. Development of antibodies to infliximab increased infliximab clearance. At 8 weeks after a maintenance dose of 3 to 10 mg/kg of infliximab, median infliximab serum concentrations ranged from approximately 0.5 to 6 mcg/mL; however, infliximab concentrations were not detectable (<0.1 mcg/mL) in patients who became positive for antibodies to infliximab. No major differences in clearance or volume of distribution were observed in patient subgroups defined by age, weight, or gender. It is not known if there are differences in clearance or volume of distribution in patients with marked impairment of hepatic or renal function.
Infliximab pharmacokinetic characteristics (including peak and trough concentrations and terminal half-life) were similar in pediatric (aged 6 to 17 years) and adult patients with Crohn's disease or ulcerative colitis following the administration of 5 mg/kg infliximab.
Population pharmacokinetic analysis showed that in children with juvenile rheumatoid arthritis (JRA) with a body weight of up to 35 kg receiving 6 mg/kg infliximab and children with JRA with body weight greater than 35 kg up to adult body weight receiving 3mg/kg infliximab, the steady state area under the concentration curve (AUCss) was similar to that observed in adults receiving 3 mg/kg of infliximab.
Nonclinical Toxicology
The significance of the results of nonclinical studies for human risk is unknown. A repeat dose toxicity study was conducted with mice given cV1q anti-mouse TNFα to evaluate tumorigenicity. CV1q is an analogous antibody that inhibits the function of TNFα in mice. Animals were assigned to 1 of 3 dose groups: control, 10 mg/kg or 40 mg/kg cV1q given weekly for 6 months. The weekly doses of 10 mg/kg and 40 mg/kg are 2 and 8 times, respectively, the human dose of 5 mg/kg for Crohn's disease. Results indicated that cV1q did not cause tumorigenicity in mice. No clastogenic or mutagenic effects of infliximab were observed in the in vivo mouse micronucleus test or the Salmonella-Escherichia coli (Ames) assay, respectively. Chromosomal aberrations were not observed in an assay performed using human lymphocytes. It is not known whether infliximab can impair fertility in humans. No impairment of fertility was observed in a fertility and general reproduction toxicity study with the analogous mouse antibody used in the 6-month chronic toxicity study.
Clinical Studies
Crohn's Disease
Active Crohn's Disease
The safety and efficacy of single and multiple doses of infliximab were assessed in 2 randomized, double-blind, placebo-controlled clinical studies in 653 patients with moderate to severely active Crohn's disease [Crohn's Disease Activity Index (CDAI) ≥220 and ≤400] with an inadequate response to prior conventional therapies. Concomitant stable doses of aminosalicylates, corticosteroids and/or immunomodulatory agents were permitted and 92% of patients continued to receive at least one of these medications.
In the single-dose trial of 108 patients, 16% (4/25) of placebo patients achieved a clinical response (decrease in CDAI ≥70 points) at Week 4 vs. 81% (22/27) of patients receiving 5 mg/kg infliximab (p<0.001, two-sided, Fisher's Exact test). Additionally, 4% (1/25) of placebo patients and 48% (13/27) of patients receiving 5 mg/kg infliximab achieved clinical remission (CDAI<150) at Week 4.
In a multidose trial (ACCENT I [Study Crohn's I]), 545 patients received 5 mg/kg at Week 0 and were then randomized to one of three treatment groups; the placebo maintenance group received placebo at Weeks 2 and 6, and then every 8 weeks; the 5 mg/kg maintenance group received 5 mg/kg at Weeks 2 and 6, and then every 8 weeks; and the 10 mg/kg maintenance group received 5 mg/kg at Weeks 2 and 6, and then 10 mg/kg every 8 weeks. Patients in response at Week 2 were randomized and analyzed separately from those not in response at Week 2. Corticosteroid taper was permitted after Week 6.
At Week 2, 57% (311/545) of patients were in clinical response. At Week 30, a significantly greater proportion of these patients in the 5 mg/kg and 10 mg/kg maintenance groups achieved clinical remission compared to patients in the placebo maintenance group (Table 3).
Additionally, a significantly greater proportion of patients in the 5 mg/kg and 10 mg/kg infliximab maintenance groups were in clinical remission and were able to discontinue corticosteroid use compared to patients in the placebo maintenance group at Week 54 (Table 3).
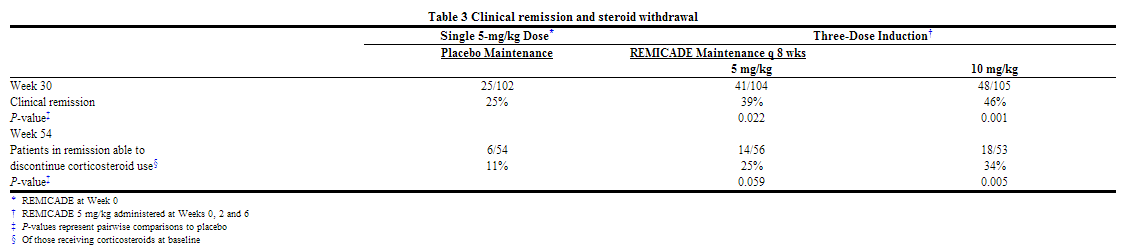
Patients in the infliximab maintenance groups (5 mg/kg and 10 mg/kg) had a longer time to loss of response than patients in the placebo maintenance group (Figure 1). At Weeks 30 and 54, significant improvement from baseline was seen among the 5 mg/kg and 10 mg/kg infliximab-treated groups compared to the placebo group in the disease-specific inflammatory bowel disease questionnaire (IBDQ), particularly the bowel and systemic components, and in the physical component summary score of the general health-related quality of life questionnaire SF-36.
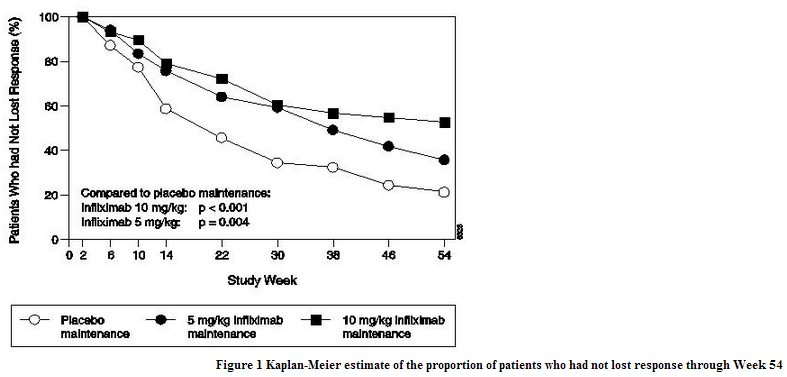
In a subset of 78 patients who had mucosal ulceration at baseline and who participated in an endoscopic substudy, 13 of 43 patients in the infliximab maintenance group had endoscopic evidence of mucosal healing compared to 1 of 28 patients in the placebo group at Week 10. Of the infliximab-treated patients showing mucosal healing at Week 10, 9 of 12 patients also showed mucosal healing at Week 54.
Patients who achieved a response and subsequently lost response were eligible to receive infliximab on an episodic basis at a dose that was 5 mg/kg higher than the dose to which they were randomized. The majority of such patients responded to the higher dose. Among patients who were not in response at Week 2, 59% (92/157) of infliximab maintenance patients responded by Week 14 compared to 51% (39/77) of placebo maintenance patients. Among patients who did not respond by Week 14, additional therapy did not result in significantly more responses.
Fistulizing Crohn's Disease
The safety and efficacy of infliximab were assessed in 2 randomized, double-blind, placebo-controlled studies in patients with fistulizing Crohn's disease with fistula(s) that were of at least 3 months duration. Concurrent use of stable doses of corticosteroids, 5-aminosalicylates, antibiotics, MTX, 6-mercaptopurine (6-MP) and/or azathioprine (AZA) was permitted.
In the first trial, 94 patients received 3 doses of either placebo or infliximab at Weeks 0, 2 and 6. Fistula response (≥50% reduction in number of enterocutaneous fistulas draining upon gentle compression on at least 2 consecutive visits without an increase in medication or surgery for Crohn's disease) was seen in 68% (21/31) of patients in the 5 mg/kg infliximab group (P=0.002) and 56% (18/32) of patients in the 10 mg/kg infliximab group (P=0.021) vs. 26% (8/31) of patients in the placebo arm. The median time to onset of response and median duration of response in infliximab-treated patients was 2 and 12 weeks, respectively. Closure of all fistulas was achieved in 52% of infliximab-treated patients compared with 13% of placebo-treated patients (P<0.001). In the second trial (ACCENT II [Study Crohn's II]), patients who were enrolled had to have at least 1 draining enterocutaneous (perianal, abdominal) fistula. All patients received 5 mg/kg infliximab at Weeks 0, 2 and 6. Patients were randomized to placebo or 5 mg/kg infliximab maintenance at Week 14. Patients received maintenance doses at Week 14 and then every 8 weeks through Week 46. Patients who were in fistula response (fistula response was defined the same as in the first trial) at both Weeks 10 and 14 were randomized separately from those not in response. The primary endpoint was time from randomization to loss of response among those patients who were in fistula response.
Among the randomized patients (273 of the 296 initially enrolled), 87% had perianal fistulas and 14% had abdominal fistulas. Eight percent also had rectovaginal fistulas. Greater than 90% of the patients had received previous immunosuppressive and antibiotic therapy.
At Week 14, 65% (177/273) of patients were in fistula response. Patients randomized to infliximab maintenance had a longer time to loss of fistula response compared to the placebo maintenance group (Figure 2). At Week 54, 38% (33/87) of infliximab-treated patients had no draining fistulas compared with 22% (20/90) of placebo-treated patients (P=0.02). Compared to placebo maintenance, patients on infliximab maintenance had a trend toward fewer hospitalizations.
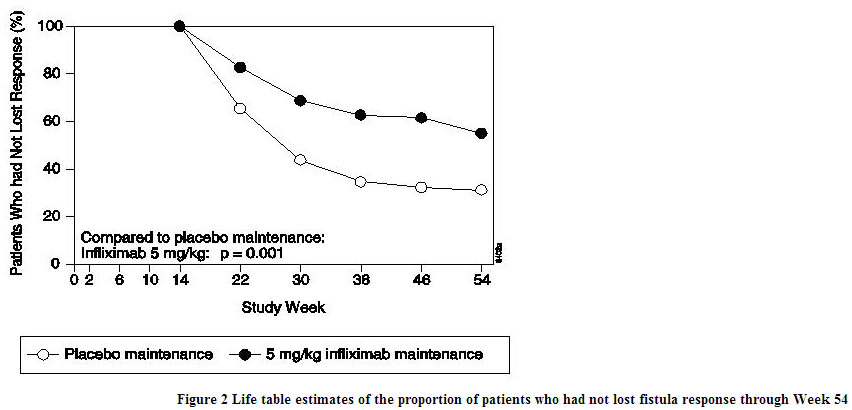
Patients who achieved a fistula response and subsequently lost response were eligible to receive infliximab maintenance therapy at a dose that was 5 mg/kg higher than the dose to which they were randomized. Of the placebo maintenance patients, 66% (25/38) responded to 5 mg/kg infliximab, and 57% (12/21) of infliximab maintenance patients responded to 10 mg/kg.
Patients who had not achieved a response by Week 14 were unlikely to respond to additional doses of infliximab.
Similar proportions of patients in either group developed new fistulas (17% overall) and similar numbers developed abscesses (15% overall).
Pediatric Crohn's Disease
The safety and efficacy of infliximab were assessed in a randomized, open-label study (Study Peds Crohn's) in 112 pediatric patients aged 6 to 17 years old with moderately to severely active Crohn's disease and an inadequate response to conventional therapies. The median age was 13 years and the median Pediatric Crohn's Disease Activity Index (PCDAI) was 40 (on a scale of 0 to 100). All patients were required to be on a stable dose of 6-MP, AZA, or MTX; 35% were also receiving corticosteroids at baseline.
All patients received induction dosing of 5 mg/kg infliximab at Weeks 0, 2, and 6. At Week 10, 103 patients were randomized to a maintenance regimen of 5 mg/kg infliximab given either every 8 weeks or every 12 weeks.
At Week 10, 88% of patients were in clinical response (defined as a decrease from baseline in the PCDAI score of ≥15 points and total PCDAI score of ≤30 points), and 59% were in clinical remission (defined as PCDAI score of ≤10 points).
The proportion of pediatric patients achieving clinical response at Week 10 compared favorably with the proportion of adults achieving a clinical response in Study Crohn's I. The study definition of clinical response in Study Peds Crohn's was based on the PCDAI score, whereas the CDAI score was used in the adult Study Crohn's I.
At both Week 30 and Week 54, the proportion of patients in clinical response was greater in the every 8-week treatment group than in the every 12-week treatment group (73% vs. 47% at Week 30, and 64% vs. 33% at Week 54). At both Week 30 and Week 54, the proportion of patients in clinical remission was also greater in the every 8-week treatment group than in the every 12-week treatment group (60% vs. 35% at Week 30, and 56% vs. 24% at Week 54), table below.
For patients in Study Peds Crohn's receiving corticosteroids at baseline, the proportion of patients able to discontinue corticosteroids while in remission at Week 30 was 46% for the every 8-week maintenance group and 33% for the every 12-week maintenance group. At Week 54, the proportion of patients able to discontinue corticosteroids while in remission was 46% for the every 8-week maintenance group and 17% for the every 12-week maintenance group.
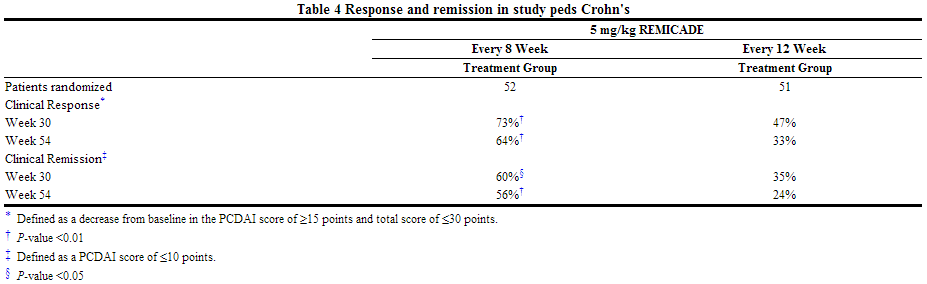
Ulcerative Colitis
The safety and efficacy of infliximab were assessed in 2 randomized, double-blind, placebo-controlled clinical studies in 728 patients with moderately to severely active ulcerative colitis (UC) (Mayo score5 6 to 12 [of possible range 0 to 12], Endoscopy subscore ≥2) with an inadequate response to conventional oral therapies (Studies UC I and UC II). Concomitant treatment with stable doses of aminosalicylates, corticosteroids and/or immunomodulatory agents was permitted. Corticosteroid taper was permitted after Week 8. Patients were randomized at week 0 to receive either placebo, 5 mg/kg infliximab or 10 mg/kg infliximab at Weeks 0, 2, 6, and every 8 weeks thereafter through Week 46 in Study UC I, and at Weeks 0, 2, 6, and every 8 weeks thereafter through Week 22 in Study UC II. In Study UC II, patients were allowed to continue blinded therapy to Week 46 at the investigator's discretion.
Patients in Study UC I had failed to respond or were intolerant to oral corticosteroids, 6-MP, or AZA. Patients in Study UC II had failed to respond or were intolerant to the above treatments and/or aminosalicylates. Similar proportions of patients in Studies UC I and UC II were receiving corticosteroids (61% and 51%, respectively), 6-MP/AZA (49% and 43%) and aminosalicylates (70% and 75%) at baseline. More patients in Study UC II than UC I were taking solely aminosalicylates for UC (26% vs. 11%, respectively). Clinical response was defined as a decrease from baseline in the Mayo score by ≥30% and ≥3 points, accompanied by a decrease in the rectal bleeding subscore of ≥1 or a rectal bleeding subscore of 0 or 1.
Clinical Response, Clinical Remission, and Mucosal Healing
In both Study UC I and Study UC II, greater percentages of patients in both infliximab groups achieved clinical response, clinical remission and mucosal healing than in the placebo group. Each of these effects was maintained through the end of each trial (Week 54 in Study UC I, and Week 30 in Study UC II). In addition, a greater proportion of patients in infliximab groups demonstrated sustained response and sustained remission than in the placebo groups (table below).
Of patients on corticosteroids at baseline, greater proportions of patients in the infliximab treatment groups were in clinical remission and able to discontinue corticosteroids at Week 30 compared with the patients in the placebo treatment groups (22% in infliximab treatment groups vs. 10% in placebo group in Study UC I; 23% in infliximab treatment groups vs. 3% in placebo group in Study UC II). In Study UC I, this effect was maintained through Week 54 (21% in infliximab treatment groups vs. 9% in placebo group). The infliximab-associated response was generally similar in the 5 mg/kg and 10 mg/kg dose groups.
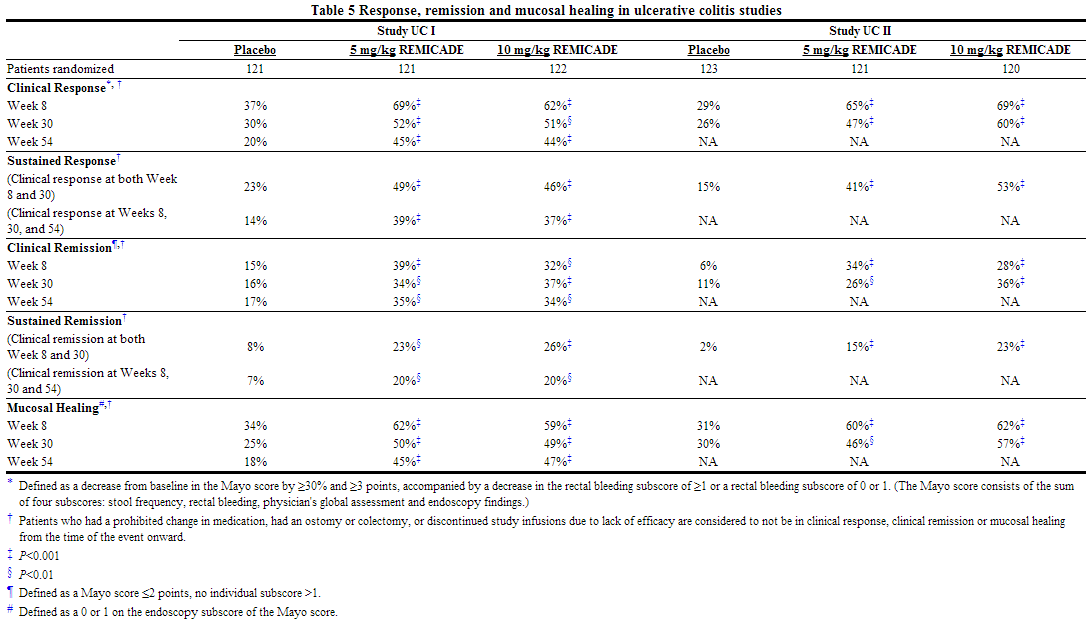
The improvement with infliximab was consistent across all Mayo subscores through Week 54 (Study UC I shown in Table 6; Study UC II through Week 30 was similar).
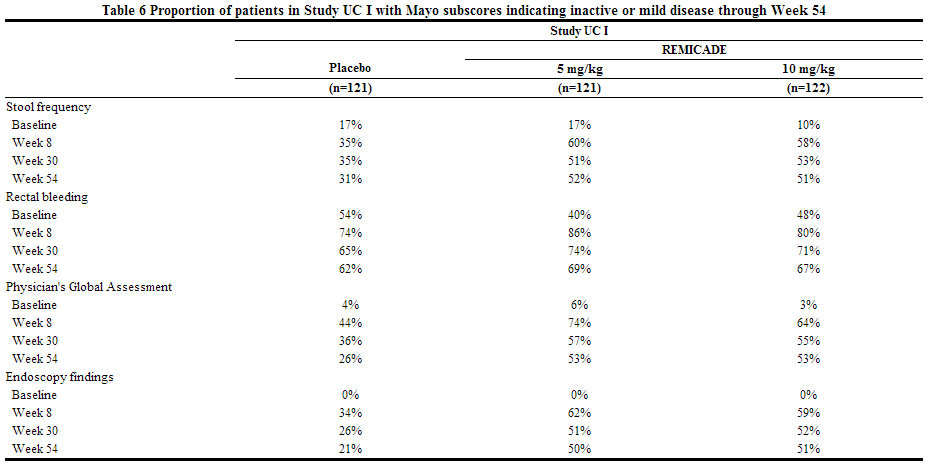
Pediatric Ulcerative Colitis
The safety and effectiveness of infliximab for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients aged 6 years and older with moderately to severely active ulcerative colitis who have had an inadequate response to conventional therapy are supported by evidence from adequate and well-controlled studies of infliximab in adults. Additional safety and pharmacokinetic data were collected in an open-label pediatric UC trial in 60 pediatric patients aged 6 through 17 years (median age 14.5 years) with moderately to severely active ulcerative colitis (Mayo score of 6 to 12; Endoscopic subscore ≥ 2) and an inadequate response to conventional therapies. At baseline, the median Mayo score was 8, 53% of patients were receiving immunomodulator therapy (6-MP/AZA/MTX), and 62% of patients were receiving corticosteroids (median dose 0.5 mg/kg/day in prednisone equivalents). Discontinuation of immunomodulators and corticosteroid taper were permitted after Week 0.
All patients received induction dosing of 5 mg/kg infliximab at Weeks 0, 2, and 6. Patients who did not respond to infliximab at Week 8 received no further infliximab and returned for safety follow-up. At Week 8, 45 patients were randomized to a maintenance regimen of 5 mg/kg infliximab given either every 8 weeks through Week 46 or every 12 weeks through Week 42. Patients were allowed to change to a higher dose and/or more frequent administration schedule if they experienced loss of response.
Clinical response at Week 8 was defined as a decrease from baseline in the Mayo score by ≥ 30% and ≥ 3 points, including a decrease in the rectal bleeding subscore by ≥ 1 points or achievement of a rectal bleeding subscore of 0 or 1.
Clinical remission at Week 8 was measured by the Mayo score, defined as a Mayo score of ≤2 points with no individual subscore >1. Clinical remission was also assessed at Week 8 and Week 54 using the Pediatric Ulcerative Colitis Activity Index (PUCAI)6 score and was defined by a PUCAI score of <10 points.
Endoscopies were performed at baseline and at Week 8. A Mayo endoscopy subscore of 0 indicated normal or inactive disease and a subscore of 1 indicated mild disease (erythema, decreased vascular pattern, or mild friability).
Of the 60 patients treated, 44 were in clinical response at Week 8. Of 32 patients taking concomitant immunomodulators at baseline, 23 achieved clinical response at Week 8, compared to 21 of 28 of those not taking concomitant immunomodulators at baseline. At Week 8, 24 of 60 patients were in clinical remission as measured by the Mayo score and 17 of 51 patients were in remission as measured by the PUCAI score.
At Week 54, 8 of 21 patients in the every 8-week maintenance group and 4 of 22 patients in the every 12-week maintenance group achieved remission as measured by the PUCAI score.
During maintenance phase, 23 of 45 randomized patients (9 in the every 8-week group and 14 in the every 12-week group) required an increase in their dose and/or increase in frequency of infliximab administration due to loss of response. Nine of the 23 patients who required a change in dose had achieved remission at Week 54. Seven of those patients received the 10 mg/kg every 8-week dosing.
Rheumatoid Arthritis
The safety and efficacy of infliximab were assessed in 2 multicenter, randomized, double-blind, pivotal trials: ATTRACT (Study RA I) and ASPIRE (Study RA II). Concurrent use of stable doses of folic acid, oral corticosteroids (≤10 mg/day) and/or non-steroidal anti-inflammatory drugs (NSAIDs) was permitted.
Study RA I was a placebo-controlled study of 428 patients with active rheumatoid arthritis despite treatment with MTX. Patients enrolled had a median age of 54 years, median disease duration of 8.4 years, median swollen and tender joint count of 20 and 31 respectively, and were on a median dose of 15 mg/wk of MTX. Patients received either placebo + MTX or one of 4 doses/schedules of infliximab + MTX: 3 mg/kg or 10 mg/kg of infliximab by IV infusion at Weeks 0, 2 and 6 followed by additional infusions every 4 or 8 weeks in combination with MTX.
Study RA II was a placebo-controlled study of 3 active treatment arms in 1004 MTX naive patients of 3 or fewer years' duration active rheumatoid arthritis. Patients enrolled had a median age of 51 years with a median disease duration of 0.6 years, median swollen and tender joint count of 19 and 31, respectively, and >80% of patients had baseline joint erosions. At randomization, all patients received MTX (optimized to 20 mg/wk by Week 8) and either placebo, 3 mg/kg or 6 mg/kg infliximab at Weeks 0, 2, and 6 and every 8 weeks thereafter.
Data on use of infliximab without concurrent MTX are limited.
Clinical response
In Study RA I, all doses/schedules of infliximab + MTX resulted in improvement in signs and symptoms as measured by the American College of Rheumatology response criteria (ACR 20) with a higher percentage of patients achieving an ACR 20, 50 and 70 compared to placebo + MTX (Table 7). This improvement was observed at Week 2 and maintained through Week 102. Greater effects on each component of the ACR 20 were observed in all patients treated with infliximab + MTX compared to placebo + MTX (Table 8). More patients treated with infliximab reached a major clinical response than placebo-treated patients (Table 7).
In Study RA II, after 54 weeks of treatment, both doses of infliximab + MTX resulted in statistically significantly greater response in signs and symptoms compared to MTX alone as measured by the proportion of patients achieving ACR 20, 50 and 70 responses (Table 7). More patients treated with infliximab reached a major clinical response than placebo-treated patients (Table 7).
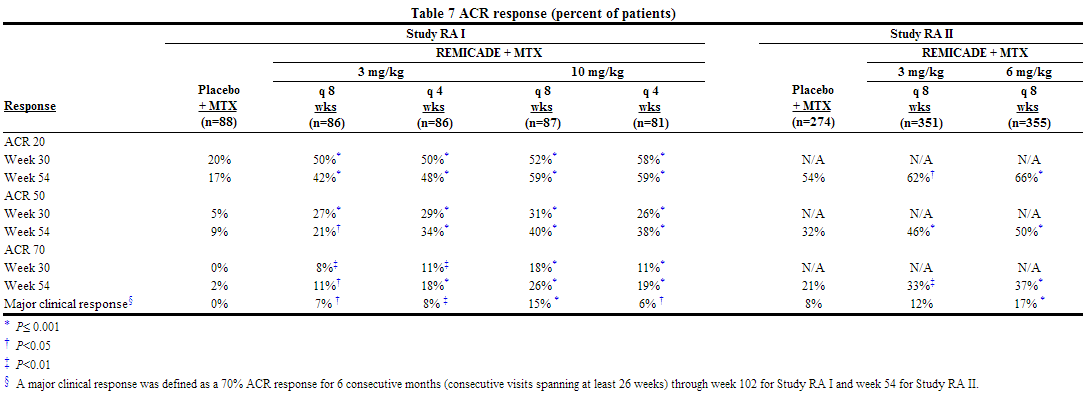
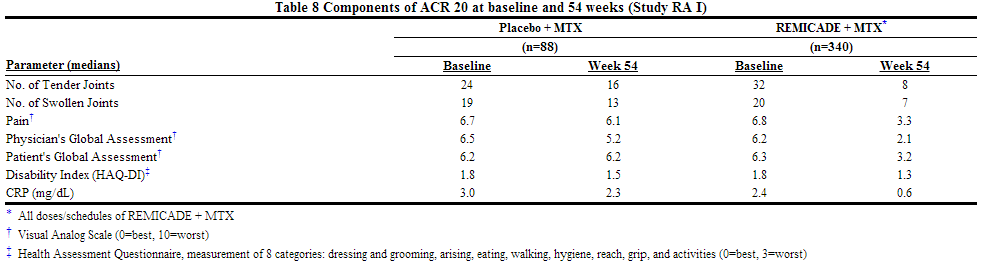
Radiographic response
Structural damage in both hands and feet was assessed radiographically at Week 54 by the change from baseline in the van der Heijde-modified Sharp (vdH-S) score, a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing in hands/wrists and feet.3
In Study RA I, approximately 80% of patients had paired X-ray data at 54 weeks and approximately 70% at 102 weeks. The inhibition of progression of structural damage was observed at 54 weeks (Table 9) and maintained through 102 weeks.
In Study RA II, >90% of patients had at least 2 evaluable X-rays. Inhibition of progression of structural damage was observed at Weeks 30 and 54 (Table 9) in the infliximab + MTX groups compared to MTX alone. Patients treated with infliximab + MTX demonstrated less progression of structural damage compared to MTX alone, whether baseline acute-phase reactants (ESR and CRP) were normal or elevated: patients with elevated baseline acute-phase reactants treated with MTX alone demonstrated a mean progression in vdH-S score of 4.2 units compared to patients treated with infliximab + MTX who demonstrated 0.5 units of progression; patients with normal baseline acute phase reactants treated with MTX alone demonstrated a mean progression in vdH-S score of 1.8 units compared to infliximab + MTX who demonstrated 0.2 units of progression. Of patients receiving infliximab + MTX, 59% had no progression (vdH-S score ≤ 0 unit) of structural damage compared to 45% of patients receiving MTX alone. In a subset of patients who began the study without erosions, infliximab + MTX maintained an erosion-free state at 1 year in a greater proportion of patients than MTX alone, 79% (77/98) vs. 58% (23/40), respectively (P<0.01). Fewer patients in the infliximab + MTX groups (47%) developed erosions in uninvolved joints compared to MTX alone (59%).
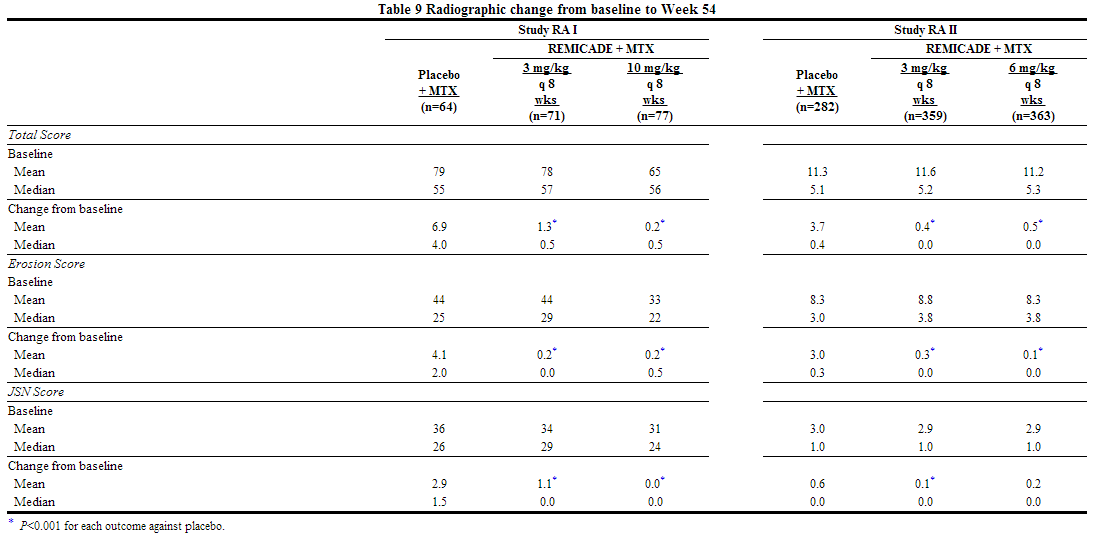
Physical function response
Physical function and disability were assessed using the Health Assessment Questionnaire (HAQ-DI) and the general health-related quality of life questionnaire SF-36.
In Study RA I, all doses/schedules of infliximab + MTX showed significantly greater improvement from baseline in HAQ-DI and SF-36 physical component summary score averaged over time through Week 54 compared to placebo + MTX, and no worsening in the SF-36 mental component summary score. The median (interquartile range) improvement from baseline to Week 54 in HAQ-DI was 0.1 (-0.1, 0.5) for the placebo + MTX group and 0.4 (0.1, 0.9) for infliximab + MTX (p<0.001). Both HAQ-DI and SF-36 effects were maintained through Week 102. Approximately 80% of patients in all doses/schedules of infliximab + MTX remained in the trial through 102 weeks.
In Study RA II, both infliximab treatment groups showed greater improvement in HAQ-DI from baseline averaged over time through Week 54 compared to MTX alone; 0.7 for infliximab + MTX vs. 0.6 for MTX alone (P≤0.001). No worsening in the SF-36 mental component summary score was observed.
Ankylosing Spondylitis
The safety and efficacy of infliximab were assessed in a randomized, multicenter, double-blind, placebo-controlled study in 279 patients with active ankylosing spondylitis. Patients were between 18 and 74 years of age, and had ankylosing spondylitis as defined by the modified New York criteria for Ankylosing Spondylitis.4 Patients were to have had active disease as evidenced by both a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score >4 (possible range 0–10) and spinal pain >4 (on a Visual Analog Scale [VAS] of 0–10). Patients with complete ankylosis of the spine were excluded from study participation, and the use of Disease Modifying Anti-Rheumatic Drugs (DMARDs) and systemic corticosteroids were prohibited. Doses of infliximab 5 mg/kg or placebo were administered intravenously at Weeks 0, 2, 6, 12 and 18.
At 24 weeks, improvement in the signs and symptoms of ankylosing spondylitis, as measured by the proportion of patients achieving a 20% improvement in ASAS response criteria (ASAS 20), was seen in 60% of patients in the infliximab-treated group vs. 18% of patients in the placebo group (p<0.001). Improvement was observed at Week 2 and maintained through Week 24 (Figure 3 and Table 10).
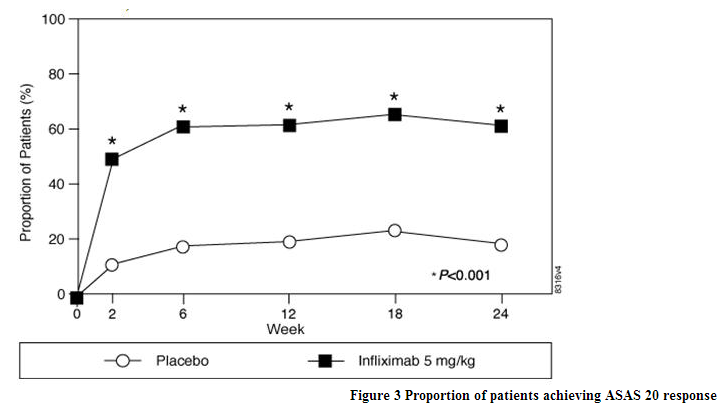
At 24 weeks, the proportions of patients achieving a 50% and a 70% improvement in the signs and symptoms of ankylosing spondylitis, as measured by ASAS response criteria (ASAS 50 and ASAS 70, respectively), were 44% and 28%, respectively, for patients receiving infliximab, compared to 9% and 4%, respectively, for patients receiving placebo (P<0.001, infliximab vs. placebo). A low level of disease activity (defined as a value <20 [on a scale of 0–100 mm] in each of the 4 ASAS response parameters) was achieved in 22% of infliximab-treated patients vs. 1% in placebo-treated patients (P<0.001).
The median improvement from baseline in the general health-related quality-of-life questionnaire SF-36 physical component summary score at Week 24 was 10.2 for the infliximab group vs. 0.8 for the placebo group (P<0.001). There was no change in the SF-36 mental component summary score in either the infliximab group or the placebo group.
Results of this study were similar to those seen in a multicenter double-blind, placebo-controlled study of 70 patients with ankylosing spondylitis.
Psoriatic Arthritis
Safety and efficacy of infliximab were assessed in a multicenter, double-blind, placebo-controlled study in 200 adult patients with active psoriatic arthritis despite DMARD or NSAID therapy (≥5 swollen joints and ≥5 tender joints) with 1 or more of the following subtypes: arthritis involving DIP joints (n=49), arthritis mutilans (n=3), asymmetric peripheral arthritis (n=40), polyarticular arthritis (n=100), and spondylitis with peripheral arthritis (n=8). Patients also had plaque psoriasis with a qualifying target lesion ≥2 cm in diameter. Forty-six percent of patients continued on stable doses of methotrexate (≤25 mg/week). During the 24-week double-blind phase, patients received either 5 mg/kg infliximab or placebo at Weeks 0, 2, 6, 14, and 22 (100 patients in each group). At Week 16, placebo patients with <10% improvement from baseline in both swollen and tender joint counts were switched to infliximab induction (early escape). At Week 24, all placebo-treated patients crossed over to infliximab induction. Dosing continued for all patients through Week 46.
Clinical response
Treatment with infliximab resulted in improvement in signs and symptoms, as assessed by the ACR criteria, with 58% of infliximab-treated patients achieving ACR 20 at Week 14, compared with 11% of placebo-treated patients (P< 0.001). The response was similar regardless of concomitant use of methotrexate. Improvement was observed as early as Week 2. At 6 months, the ACR 20/50/70 responses were achieved by 54%, 41%, and 27%, respectively, of patients receiving infliximab compared to 16%, 4%, and 2%, respectively, of patients receiving placebo. Similar responses were seen in patients with each of the subtypes of psoriatic arthritis, although few patients were enrolled with the arthritis mutilans and spondylitis with peripheral arthritis subtypes.
Compared to placebo, treatment with infliximab resulted in improvements in the components of the ACR response criteria, as well as in dactylitis and enthesopathy (Table 11). The clinical response was maintained through Week 54. Similar ACR responses were observed in an earlier randomized, placebo-controlled study of 104 psoriatic arthritis patients, and the responses were maintained through 98 weeks in an open-label extension phase.
Improvement in Psoriasis Area and Severity Index (PASI) in psoriatic arthritis patients with baseline body surface area (BSA) ≥3% (n=87 placebo, n=83 infliximab) was achieved at Week 14, regardless of concomitant methotrexate use, with 64% of infliximab-treated patients achieving at least 75% improvement from baseline vs. 2% of placebo-treated patients; improvement was observed in some patients as early as Week 2. At 6 months, the PASI 75 and PASI 90 responses were achieved by 60% and 39%, respectively, of patients receiving infliximab compared to 1% and 0%, respectively, of patients receiving placebo. The PASI response was generally maintained through Week 54.
Radiographic response
Structural damage in both hands and feet was assessed radiographically by the change from baseline in the van der Heijde-Sharp (vdH-S) score, modified by the addition of hand DIP joints. The total modified vdH-S score is a composite score of structural damage that measures the number and size of joint erosions and the degree of joint space narrowing (JSN) in the hands and feet. At Week 24, infliximab-treated patients had less radiographic progression than placebo-treated patients (mean change of -0.70 vs. 0.82, P<0.001). infliximab-treated patients also had less progression in their erosion scores (-0.56 vs 0.51) and JSN scores (-0.14 vs 0.31). The patients in the infliximab group demonstrated continued inhibition of structural damage at Week 54. Most patients showed little or no change in the vdH-S score during this 12-month study (median change of 0 in both patients who initially received infliximab or placebo). More patients in the placebo group (12%) had readily apparent radiographic progression compared with the infliximab group (3%).
Physical function
Physical function status was assessed using the HAQ Disability Index (HAQ-DI) and the SF-36 Health Survey. infliximab-treated patients demonstrated significant improvement in physical function as assessed by HAQ-DI (median percent improvement in HAQ-DI score from baseline to Week 14 and 24 of 43% for infliximab-treated patients vs 0% for placebo-treated patients).
During the placebo-controlled portion of the trial (24 weeks), 54% of infliximab-treated patients achieved a clinically meaningful improvement in HAQ-DI (≥0.3 unit decrease) compared to 22% of placebo-treated patients. infliximab-treated patients also demonstrated greater improvement in the SF-36 physical and mental component summary scores than placebo-treated patients. The responses were maintained for up to 2 years in an open-label extension study.
Plaque Psoriasis
The safety and efficacy of infliximab were assessed in 3 randomized, double-blind, placebo-controlled studies in patients 18 years of age and older with chronic, stable plaque psoriasis involving ≥10% BSA, a minimum PASI score of 12, and who were candidates for systemic therapy or phototherapy. Patients with guttate, pustular, or erythrodermic psoriasis were excluded from these studies. No concomitant anti-psoriatic therapies were allowed during the study, with the exception of low-potency topical corticosteroids on the face and groin after Week 10 of study initiation.
Study I (EXPRESS) evaluated 378 patients who received placebo or infliximab at a dose of 5 mg/kg at Weeks 0, 2, and 6 (induction therapy), followed by maintenance therapy every 8 weeks. At Week 24, the placebo group crossed over to infliximab induction therapy (5 mg/kg), followed by maintenance therapy every 8 weeks. Patients originally randomized to infliximab continued to receive infliximab 5 mg/kg every 8 weeks through Week 46. Across all treatment groups, the median baseline PASI score was 21 and the baseline Static Physician Global Assessment (sPGA) score ranged from moderate (52% of patients) to marked (36%) to severe (2%). In addition, 75% of patients had a BSA >20%. Seventy-one percent of patients previously received systemic therapy, and 82% received phototherapy.
Study II (EXPRESS II) evaluated 835 patients who received placebo or infliximab at doses of 3 mg/kg or 5 mg/kg at Weeks 0, 2, and 6 (induction therapy). At Week 14, within each infliximab dose group, patients were randomized to either scheduled (every 8 weeks) or as needed (PRN) maintenance treatment through Week 46. At Week 16, the placebo group crossed over to infliximab induction therapy (5 mg/kg), followed by maintenance therapy every 8 weeks. Across all treatment groups, the median baseline PASI score was 18, and 63% of patients had a BSA >20%. Fifty-five percent of patients previously received systemic therapy, and 64% received a phototherapy.
Study III (SPIRIT) evaluated 249 patients who had previously received either psoralen plus ultraviolet A treatment (PUVA) or other systemic therapy for their psoriasis. These patients were randomized to receive either placebo or infliximab at doses of 3 mg/kg or 5 mg/kg at Weeks 0, 2, and 6. At Week 26, patients with a sPGA score of moderate or worse (greater than or equal to 3 on a scale of 0 to 5) received an additional dose of the randomized treatment. Across all treatment groups, the median baseline PASI score was 19, and the baseline sPGA score ranged from moderate (62% of patients) to marked (22%) to severe (3%). In addition, 75% of patients had a BSA >20%. Of the enrolled patients, 114 (46%) received the Week 26 additional dose.
In Studies I, II and III, the primary endpoint was the proportion of patients who achieved a reduction in score of at least 75% from baseline at Week 10 by the PASI (PASI 75). In Study I and Study III, another evaluated outcome included the proportion of patients who achieved a score of "cleared" or "minimal" by the sPGA. The sPGA is a 6-category scale ranging from "5 = severe" to "0 = cleared" indicating the physician's overall assessment of the psoriasis severity focusing on induration, erythema, and scaling. Treatment success, defined as "cleared" or "minimal," consisted of none or minimal elevation in plaque, up to faint red coloration in erythema, and none or minimal fine scale over <5% of the plaque.
Study II also evaluated the proportion of patients who achieved a score of "clear" or "excellent" by the relative Physician's Global Assessment (rPGA). The rPGA is a 6-category scale ranging from "6 = worse" to "1 = clear" that was assessed relative to baseline. Overall lesions were graded with consideration to the percent of body involvement as well as overall induration, scaling, and erythema. Treatment success, defined as "clear" or "excellent," consisted of some residual pinkness or pigmentation to marked improvement (nearly normal skin texture; some erythema may be present). The results of these studies are presented in Table 12.
In Study I, in the subgroup of patients with more extensive psoriasis who had previously received phototherapy, 85% of patients on 5 mg/kg infliximab achieved a PASI 75 at Week 10 compared with 4% of patients on placebo.
In Study II, in the subgroup of patients with more extensive psoriasis who had previously received phototherapy, 72% and 77% of patients on 3 mg/kg and 5 mg/kg infliximab achieved a PASI 75 at Week 10 respectively compared with 1% on placebo. In Study II, among patients with more extensive psoriasis who had failed or were intolerant to phototherapy, 70% and 78% of patients on 3 mg/kg and 5 mg/kg infliximab achieved a PASI 75 at Week 10 respectively, compared with 2% on placebo.
Maintenance of response was studied in a subset of 292 and 297 infliximab-treated patients in the 3 mg/kg and 5 mg/kg groups; respectively, in Study II. Stratified by PASI response at Week 10 and investigational site, patients in the active treatment groups were re-randomized to either a scheduled or as needed maintenance (PRN) therapy, beginning on Week 14.
The groups that received a maintenance dose every 8 weeks appear to have a greater percentage of patients maintaining a PASI 75 through week 50 as compared to patients who received the as-needed or PRN doses, and the best response was maintained with the 5 mg/kg every 8-week dose. These results are shown in Figure 4. At Week 46, when infliximab serum concentrations were at trough level, in the every 8-week dose group, 54% of patients in the 5 mg/kg group compared to 36% in the 3 mg/kg group achieved PASI 75. The lower percentage of PASI 75 responders in the 3 mg/kg every 8-week dose group compared to the 5 mg/kg group was associated with a lower percentage of patients with detectable trough serum infliximab levels. This may be related in part to higher antibody rates. In addition, in a subset of patients who had achieved a response at Week 10, maintenance of response appears to be greater in patients who received infliximab every 8 weeks at the 5 mg/kg dose. Regardless of whether the maintenance doses are PRN or every 8 weeks, there is a decline in response in a subpopulation of patients in each group over time. The results of Study I through Week 50 in the 5 mg/kg every 8 weeks maintenance dose group were similar to the results from Study II.
Efficacy and safety of infliximab treatment beyond 50 weeks have not been evaluated in patients with plaque psoriasis.
How Supplied
Each infliximab 20 mL vial is individually packaged in a carton. Infliximab is supplied in an accumulator carton containing 10 vials.
- NDC 57894-030-01 100 mg vial
- Each single dose vial contains 100 mg of infliximab for final reconstitution volume of 10 mL.
Storage
- Infliximab must be refrigerated at 2ºC to 8ºC (36ºF to 46ºF).
- Do not use infliximab beyond the expiration date (Exp) located on the carton and the vial. This product contains no preservative.
Images
Drug Images
{{#ask: Page Name::Infliximab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Infliximab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients or their caregivers should be advised of the potential benefits and risks of infliximab. Physicians should instruct their patients to read the Medication Guide before starting infliximab therapy and to reread it each time they receive an infusion. It is important that the patient's overall health be assessed at each treatment visit and that any questions resulting from the patient's or their caregiver's reading of the Medication Guide be discussed.
- Immunosuppression: Inform patients that infliximab may lower the ability of their immune system to fight infections. Instruct patients of the importance of contacting their doctors if they develop any symptoms of an infection, including tuberculosis and reactivation of hepatitis B virus infections. Patients should be counseled about the risk of lymphoma and other malignancies while receiving infliximab.
- Other Medical Conditions: Advise patients to report any signs of new or worsening medical conditions such as heart disease, neurological disease, or autoimmune disorders. Advise patients to report any symptoms of a cytopenia such as bruising, bleeding or persistent fever.
Precautions with Alcohol
Alcohol-Infliximab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Remicade
Look-Alike Drug Names
- Infliximab - Rituximab
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Kraetsch HG, Antoni C, Kalden JR, Manger B (2001). "Successful treatment of a small cohort of patients with adult onset of Still's disease with infliximab: first experiences". Ann Rheum Dis. 60 Suppl 3: iii55–7. PMC 1766667. PMID 11890655.
- ↑ Cavagna L, Caporali R, Epis O, Bobbio-Pallavicini F, Montecucco C (2001). "Infliximab in the treatment of adult Still's disease refractory to conventional therapy". Clin Exp Rheumatol. 19 (3): 329–32. PMID 11407090.
- ↑ Sullivan TP, Welsh E, Kerdel FA, Burdick AE, Kirsner RS (2003). "Infliximab for hidradenitis suppurativa". Br J Dermatol. 149 (5): 1046–9. PMID 14632813.
- ↑ Elliott MJ, Maini RN, Feldmann M, Kalden JR, Antoni C, Smolen JS; et al. (1994). "Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis". Lancet. 344 (8930): 1105–10. PMID 7934491.
- ↑ Quinn MA, Conaghan PG, O'Connor PJ, Karim Z, Greenstein A, Brown A; et al. (2005). "Very early treatment with infliximab in addition to methotrexate in early, poor-prognosis rheumatoid arthritis reduces magnetic resonance imaging evidence of synovitis and damage, with sustained benefit after infliximab withdrawal: results from a twelve-month randomized, double-blind, placebo-controlled trial". Arthritis Rheum. 52 (1): 27–35. doi:10.1002/art.20712. PMID 15641102.
- ↑ Mekinian A, Néel A, Sibilia J, Cohen P, Connault J, Lambert M; et al. (2012). "Efficacy and tolerance of infliximab in refractory Takayasu arteritis: French multicentre study". Rheumatology (Oxford). 51 (5): 882–6. doi:10.1093/rheumatology/ker380. PMID 22223706.
- ↑ Tynjälä P, Lindahl P, Honkanen V, Lahdenne P, Kotaniemi K (2007). "Infliximab and etanercept in the treatment of chronic uveitis associated with refractory juvenile idiopathic arthritis". Ann Rheum Dis. 66 (4): 548–50. doi:10.1136/ard.2006.058248. PMC 1856052. PMID 17068061.
- ↑ Bartolucci P, Ramanoelina J, Cohen P, Mahr A, Godmer P, Le Hello C; et al. (2002). "Efficacy of the anti-TNF-alpha antibody infliximab against refractory systemic vasculitides: an open pilot study on 10 patients". Rheumatology (Oxford). 41 (10): 1126–32. PMID 12364631.
{{#subobject:
|Label Page=Infliximab |Label Name=InfliximabPackage.png
}}
Template:Immunosuppressants Template:Chimericmonoclonals de:Infliximab he:רמיקייד Template:Jb1