Idelalisib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, AND INTESTINAL PERFORATION
See full prescribing information for complete Boxed Warning.
Condition Name:
|
Overview
Idelalisib is an antineoplastic agent that is FDA approved for the treatment of relapsed chronic lymphocytic leukemia, relapsed follicular b-cell non-hodgkin lymphoma and relapsed small lymphocytic lymphoma. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hyperglycemia, hypertriglyceridemia, abdominal pain, nausea, neutropenia, cough, fatigue, fever and shivering.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Dose
- The recommended maximum starting dose of Zydelig is 150 mg administered orally twice daily.
- Zydelig can be taken with or without food. Tablets should be swallowed whole.
- Continue treatment until disease progression or unacceptable toxicity. The optimal and safe dosing regimen for patients who receive treatment longer than several months is unknown.
Dose Modification
- See the table below for dose modification instructions for specific toxicities related to Zydelig.
- For other severe or life-threatening toxicities related to Zydelig, withhold drug until toxicity is resolved. If resuming Zydelig after interruption for other severe or life-threatening toxicities, reduce the dose to 100 mg twice daily. Recurrence of other severe or life-threatening Zydelig-related toxicity upon rechallenge should result in permanent discontinuation of Zydelig.

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Idelalisib in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Idelalisib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Idelalisib FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Idelalisib in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Idelalisib in pediatric patients.
Contraindications
- History of serious allergic reactions including anaphylaxis and toxic epidermal necrolysis.
Warnings
|
FATAL AND SERIOUS TOXICITIES: HEPATIC, SEVERE DIARRHEA, COLITIS, PNEUMONITIS, AND INTESTINAL PERFORATION
See full prescribing information for complete Boxed Warning.
Condition Name:
|
Hepatotoxicity
- Fatal and/or serious hepatotoxicity occurred in 14% of patients treated with Zydelig. Elevations in ALT or AST greater than 5 times the upper limit of normal have occurred. These findings were generally observed within the first 12 weeks of treatment and were reversible with dose interruption. After resumption of treatment at a lower dose, 26% of patients had recurrence of ALT and AST elevations. Discontinue Zydelig for recurrent hepatotoxicity.
- Avoid concurrent use of Zydelig with other drugs that may cause liver toxicity.
- Monitor ALT and AST in all patients every 2 weeks for the first 3 months of treatment, every 4 weeks for the next 3 months, then every 1 to 3 months thereafter. Monitor weekly for liver toxicity if the ALT or AST rises above 3 times the upper limit of normal until resolved. Withhold Zydelig if the ALT or AST is greater than 5 times the upper limit of normal, and continue to monitor AST, ALT and total bilirubin weekly until the abnormality is resolved.
Severe Diarrhea or Colitis
- Severe diarrhea or colitis (Grade 3 or higher) occurred in 14% of Zydelig-treated patients across clinical trials. Diarrhea can occur at any time. Avoid concurrent use of Zydelig and other drugs that cause diarrhea. Diarrhea due to Zydelig responds poorly to antimotility agents. Median time to resolution ranged between 1 week and 1 month across trials, following interruption of Zydelig therapy and in some instances, use of corticosteroids.
Pneumonitis
- Fatal and serious pneumonitis occurred in patients treated with Zydelig. Patients taking Zydelig who present with pulmonary symptoms such as cough, dyspnea, hypoxia, interstitial infiltrates on a radiologic exam, or a decline by more than 5% in oxygen saturation should be evaluated for pneumonitis. If pneumonitis is suspected, interrupt Zydelig until the etiology of the pulmonary symptoms has been determined. Patients with pneumonitis thought to be caused by Zydelig have been treated with discontinuation of Zydelig and administration of corticosteroids.
Intestinal Perforation
- Fatal and serious intestinal perforation occurred in Zydelig-treated patients. At the time of perforation, some patients had moderate to severe diarrhea. Advise patients to promptly report any new or worsening abdominal pain, chills, fever, nausea, or vomiting. Discontinue Zydelig permanently in patients who experience intestinal perforation.
Severe Cutaneous Reactions
- One case of toxic epidermal necrolysis (TEN) occurred in a study of Zydelig in combination with rituximab and bendamustine. Other severe or life-threatening (Grade ≥3) cutaneous reactions, including dermatitis exfoliative, rash, erythematous rash, generalized rash, macular rash, maculo-papular rash, papular rash, pruritic rash, exfoliative rash, and skin disorder, have been reported in Zydelig-treated patients. Monitor patients for the development of severe cutaneous reactions and discontinue Zydelig.
Anaphylaxis
- Serious allergic reactions, including anaphylaxis, have been reported in patients on Zydelig. In patients who develop serious allergic reactions, discontinue Zydelig permanently and institute appropriate supportive measures.
Neutropenia
- Treatment-emergent Grade 3 or 4 neutropenia occurred in 31% of Zydelig-treated patients across clinical trials. Monitor blood counts at least every two weeks for the first 3 months of therapy, and at least weekly in patients while neutrophil counts are less than 1.0 Gi/L.
Embryo-fetal Toxicity
- Based on findings in animals, Zydelig may cause fetal harm when administered to a pregnant woman. Idelalisib is teratogenic in rats, at systemic exposures 12 times those reported in patients at the recommended dose of 150 mg twice daily. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
- Advise females of reproductive potential to avoid becoming pregnant while taking Zydelig. If contraceptive methods are being considered, use effective contraception during treatment, and for at least 1 month after the last dose of Zydelig.
Adverse Reactions
Clinical Trials Experience
Summary of Clinical Trials in Chronic Lymphocytic Leukemia
- The safety data reflect subject exposure to Zydelig from Study 1, in which 218 subjects with relapsed CLL received up to 8 doses of rituximab with or without Zydelig 150 mg twice daily. The median duration of exposure to Zydelig was 5 months.
- Serious adverse reactions were reported in 54 (49%) subjects treated with Zydelig + rituximab. The most frequent (≥2%) serious adverse reactions reported for subjects treated with Zydelig were pneumonia (17%), pyrexia (9%), sepsis (8%), febrile neutropenia (5%) and diarrhea (5%). Adverse reactions that led to discontinuation of Zydelig occurred in 11 (10%) subjects. The most common adverse reactions that led to treatment discontinuations were hepatotoxicity and diarrhea/colitis.
- Thirty-nine subjects (35%) had dose interruptions and sixteen subjects (15%) had dose reductions due to adverse reactions or laboratory abnormalities. The most common reasons for dose reductions were elevated transaminases, diarrhea or colitis, and rash.
- Table 2 and Table 3 summarize common adverse reactions and laboratory abnormalities reported for Zydelig + rituximab and placebo + rituximab arms.

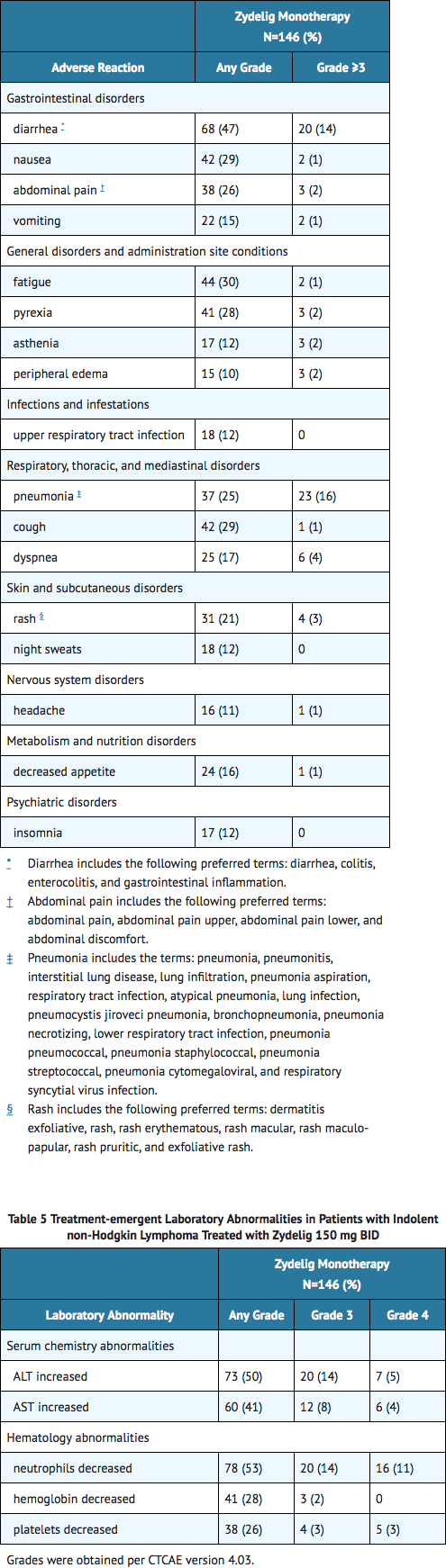
Summary of Clinical Trials in Indolent Non-Hodgkin Lymphoma
- The safety data reflect exposure to Zydelig in 146 adults with indolent non-Hodgkin lymphoma treated with Zydelig 150 mg twice daily in clinical trials. The median duration of exposure was 6.1 months (range 0.3 to 26.4 months).
- Serious adverse reactions were reported in 73 (50%) subjects. The most frequent serious adverse reactions that occurred were pneumonia (15%), diarrhea (11%), and pyrexia (9%).
- Adverse reactions resulted in interruption or discontinuation for 78 (53%) subjects. The most common reasons for interruption or discontinuations were diarrhea (11%), pneumonia (11%), and elevated transaminases (10%).
Table 4 provides the adverse reactions occurring in at least 10% of subjects receiving Zydelig monotherapy, and Table 5 provides the treatment-emergent laboratory abnormalities.
Postmarketing Experience
There is limited information regarding Idelalisib Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Idelalisib Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Idelalisib in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Idelalisib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Idelalisib during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Idelalisib in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Idelalisib in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Idelalisib in geriatric settings.
Gender
There is no FDA guidance on the use of Idelalisib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Idelalisib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Idelalisib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Idelalisib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Idelalisib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Idelalisib in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Idelalisib Administration in the drug label.
Monitoring
There is limited information regarding Idelalisib Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Idelalisib and IV administrations.
Overdosage
There is limited information regarding Idelalisib overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Idelalisib Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Idelalisib Mechanism of Action in the drug label.
Structure
There is limited information regarding Idelalisib Structure in the drug label.
Pharmacodynamics
There is limited information regarding Idelalisib Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Idelalisib Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Idelalisib Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Idelalisib Clinical Studies in the drug label.
How Supplied
There is limited information regarding Idelalisib How Supplied in the drug label.
Storage
There is limited information regarding Idelalisib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Idelalisib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Idelalisib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Idelalisib Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Idelalisib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Idelalisib Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Idelalisib Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.