Gadoversetamide: Difference between revisions
Adeel Jamil (talk | contribs) No edit summary |
Adeel Jamil (talk | contribs) No edit summary |
||
| Line 6: | Line 6: | ||
|indicationType=diagnosis | |indicationType=diagnosis | ||
|adverseReactions=vasodilatation, sensation of hot and cold, nausea, taste sense altered, dizziness, headache and paresthesia | |adverseReactions=vasodilatation, sensation of hot and cold, nausea, taste sense altered, dizziness, headache and paresthesia | ||
|blackBoxWarningTitle= | |blackBoxWarningTitle=WARNING: NEPHROGENIC SYSTEMIC FIBROSIS | ||
|blackBoxWarningBody= | |blackBoxWarningBody=* Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrasted MRI or other modalities. NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. | ||
* Do not administer Optimark to patients with: | |||
:* chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or | |||
:* acute kidney injury [see Contraindications (4)]. | |||
* Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. | |||
* Do not exceed the recommended Optimark dose and allow a sufficient period of time for elimination of the drug from the body prior to any re-administration [see Warnings and Precautions | |||
|fdaLIADAdult======MRI of Central Nervous System (CNS)===== | |fdaLIADAdult======MRI of Central Nervous System (CNS)===== | ||
Revision as of 15:46, 1 April 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Gadoversetamide is a diagnostic agent and radiological contrast media that is FDA approved for the diagnosis of {{{indication}}}. Common adverse reactions include vasodilatation, sensation of hot and cold, nausea, taste sense altered, dizziness, headache and paresthesia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
MRI of Central Nervous System (CNS)
- Optimark is indicated for use with magnetic resonance imaging (MRI) in patients with abnormal blood-brain barrier or abnormal vascularity of the brain, spine and associated tissues.
MRI of Liver
- Optimark is indicated for use with MRI to provide contrast enhancement and facilitate visualization of lesions with abnormal vascularity in the liver of patients who are highly suspect for liver structural abnormalities on computed tomography.
Dosing Guidelines
- Administer Optimark as a bolus peripheral intravenous injection at a dose of 0.2 mL/kg (0.1 mmol/kg) and at a rate of 1 to 2 mL/sec delivered by manual or by power injection (see Table 1).
- Use sterile technique to withdraw and administer Optimark.
- Follow injection with a 5 mL normal saline flush to ensure complete administration of the contrast.
- Discard unused portions of the drug.
t
Drug Handling
- Visually inspect Optimark for particulate matter and discoloration prior to administration. Do not use the solution if it is discolored or particulate matter is present.
- Do not mix Optimark with other medications or parenteral nutrition and do not administer Optimark in the same intravenous line as other medications because of the potential for chemical incompatibility.
Imaging
- Complete the imaging procedure within 1 hour of the injection of Optimark.
Paramagnetic contrast agents may impair the visualization of lesions seen on non-contrast MRI. Interpret Optimark MR images with companion non-contrast MR images.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gadoversetamide in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gadoversetamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Gadoversetamide FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Gadoversetamide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Gadoversetamide in pediatric patients.
Contraindications
- Optimark is contraindicated in patients with:
- Chronic, severe kidney disease (glomerular filtration rate, GFR <30 mL/min/1.73m2)
acute kidney injury
- Known hypersensitivity reactions to gadolinium, versetamide or any of the inert ingredients.
Warnings
Nephrogenic Systemic Fibrosis (NSF)
- Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs among these patients unless the diagnostic information is essential and not available with non-contrast enhanced MRI or other modalities. The GBCA-associated NSF risk appears highest for patients with chronic, severe kidney disease (GFR <30 mL/min/1.73m2) as well as patients with acute kidney injury. Do not administer Optimark to these patients. The risk appears lower for patients with chronic, moderate kidney disease (GFR 30 to 59 mL/min/1.73m2) and little, if any, for patients with chronic, mild kidney disease (GFR 60 to 89 mL/min/1.73m2). NSF may result in fatal or debilitating fibrosis affecting the skin, muscle and internal organs. Report any diagnosis of NSF following Optimark administration to Mallinckrodt Inc. (1‑800‑778‑7898) or FDA (1-800-FDA-1088 or www.fda.gov/medwatch).
- Screen patients for acute kidney injury and other conditions that may reduce renal function. Features of acute kidney injury consist of rapid (over hours to days) and usually reversible decrease in kidney function, commonly in the setting of surgery, severe infection, injury, or drug-induced kidney toxicity. Serum creatinine levels and estimated GFR may not reliably assess renal function in the setting of acute kidney injury. For patients at risk for chronic kidney disease (e.g., age >60 years, diabetes mellitus or chronic hypertension), estimate the GFR through laboratory testing.
- Among the factors that may increase the risk for NSF are repeated or higher than recommended doses of a GBCA and the degree of renal impairment at the time of exposure. Record the specific GBCA and the dose administered to a patient. When administering Optimark, do not exceed the recommended dose and allow a sufficient period of time for elimination of the drug prior to any re-administration.
Acute Kidney Injury (AKI)
- In patients with chronically reduced renal function, acute kidney injury requiring dialysis has occurred with the use of GBCAs. The risk of acute kidney injury may increase with increasing dose of the contrast agent; administer the lowest dose necessary for adequate imaging.
Hypersensitivity Reactions
- Severe hypersensitivity reactions including anaphylaxis have been observed with administration of gadolinium products including Optimark. Before administering Optimark ensure the availability of resuscitation equipment and personnel trained in resuscitation techniques. Patients with a history of allergy, drug reactions or other hypersensitivity-like disorders may be at greater risk and should be closely observed during the procedure and for several hours after drug administration. If a reaction occurs, stop Optimark and immediately begin appropriate therapy including resuscitation.
Interference with Laboratory Testing
- Interference by Optimark in the measurements of serum iron, copper and zinc has been observed. Optimark causes interference in the measurement of serum calcium using the ortho-cresophthalin complexone (OCP) colorimetric method. In the presence of Optimark, OCP produces an erroneous, low value for serum calcium. The magnitude of this artifact is proportional to the concentration of Optimark in the blood, and accurate values can be obtained approximately 90 minutes following injection. In patients with renal insufficiency, clearance of Optimark is slowed and the interference with calcium determination by OCP is prolonged. Neither the arsenazo III dye system nor the inductively coupled plasma mass spectroscopy methods for calcium assay are affected by Optimark.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the prescribing information:
- Nephrogenic Systemic Fibrosis
- Hypersensitivity reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The adverse reactions described in this section were observed in a total of 1,309 subjects (24 healthy volunteers and 1,285 patients in clinical trials). Patients ranged in age from 12 to 85 years (mean age of 50 years) and 680 subjects (52%) were men. The ethnic distribution was 84% White, 9% Black, 3% Asian, and 4% other.
- Overall, 460 subjects (35%) reported at least one adverse reaction. Most adverse reactions were mild or moderate in severity. The most commonly noted adverse reactions were: injection associated discomfort (26%), headache (9.4%), vasodilatation (6.4%), taste perversion (6.2%), dizziness (3.7%), nausea (3.2%), and paresthesia (2.2%). Table 2 lists adverse reactions reported in 1% or greater of patients.
T
- The following adverse reactions occurred in less than 1% of the patients:
Body as a Whole:
- Allergic reaction, facial edema, fever, malaise, neck rigidity, neck pain, pelvic pain, increased sweating
Cardiovascular:
- Arrhythmia, chest pain, hypertension, hypotension, pallor, palpitation, syncope, tachycardia, vasospasm
Digestive:
- Anorexia, constipation, dry mouth, dysphagia, eructation, increased salivation, thirst, vomiting
Metabolic and Nutritional:
- Increased creatinine, edema, hypercalcemia
Musculoskeletal:
- Arthralgia, leg cramps, myalgia, spasm
Nervous System:=
- Agitation, anxiety, confusion, diplopia, dystonia, hypertonia, hypesthesia, somnolence, tremor, vertigo
Respiratory System:
Cough, dyspnea, laryngismus, pharyngitis, sinusitis, voice alteration
Skin and Appendages:
- Erythema multiforme, pruritus, rash, thrombophlebitis, urticaria
Special Senses:=
- Parosmia, tinnitus
Urogenital:=
- Oliguria
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of Optimark. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to Optimark.
- Nephrogenic Systemic Fibrosis (NSF)
- Hypersensitivity reactions including bronchospasm and laryngeal/pharyngeal edema
- Seizures
Drug Interactions
There is limited information regarding Gadoversetamide Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
There is no FDA guidance on usage of Gadoversetamide in women who are pregnant.
Pregnancy Category (AUS):
- There are no adequate and well-controlled studies in pregnant women. Optimark should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Gadoversetamide administered to rats reduced neonatal weights from birth through weaning at maternal doses of 0.5 mmol/kg/day (1 times the human dose based on body surface area) for 5 weeks (including gestation) and paternal doses of 0.5 mmol/kg/day for 12 weeks. This effect was not observed at 0.1 mmol/kg (0.2 times the human dose based on body surface area). Maternal toxicity was not observed at any dose.
- Gadoversetamide injection caused a reduced mean fetal weight, abnormal liver lobation, delayed ossification of sternebrae, and delayed behavioral development (startle reflex and air righting reflex) in fetuses from female rats dosed with 4.9 mmol/kg/day (10 times the human dose based on body surface area) on days 7 through 17 of gestation. These effects were not observed at 0.7 mmol/kg/day (1 times the human dose based on body surface area). Maternal toxicity was observed at 4.9 mmol/kg/day.
- Gadoversetamide injection caused forelimb flexures and cardiovascular changes in fetuses from female rabbits dosed with 0.4 and 1.6 mmol/kg/day (respectively, 1 and 4 times the human dose based on body surface area) on gestation days 6 through 18. The cardiovascular changes were malformed thoracic arteries, a septal defect, and abnormal ventricle. These effects were not observed at 0.1 mmol/kg/day (0.3 times the human dose based on body surface area). Maternal toxicity was not observed at any dose.
Labor and Delivery
There is no FDA guidance on use of Gadoversetamide during labor and delivery.
Nursing Mothers
- Radiolabeled gadoversetamide (153Gd) was excreted in the milk of lactating rats receiving a single intravenous dose of 0.1 mmol/kg. Women should discontinue nursing and discard breast milk up to 72 hours after Optimark administration
Pediatric Use
- The safety and effectiveness of Optimark in pediatric patients have not been established. Pediatric patients may be particularly vulnerable to adverse GBCA reactions due to renal immaturity or unrecognized renal insufficiency.
Geriatic Use
There is no FDA guidance on the use of Gadoversetamide in geriatric settings.
Gender
There is no FDA guidance on the use of Gadoversetamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Gadoversetamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Gadoversetamide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Gadoversetamide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Gadoversetamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Gadoversetamide in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Gadoversetamide Administration in the drug label.
Monitoring
There is limited information regarding Gadoversetamide Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Gadoversetamide and IV administrations.
Overdosage
There is limited information regarding Gadoversetamide overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
| |
Gadoversetamide
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | V08 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 661.77 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | Nil |
| Metabolism | Nil |
| Half life | 80 to 120 minutes |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | Intravenous |
Mechanism of Action
There is limited information regarding Gadoversetamide Mechanism of Action in the drug label.
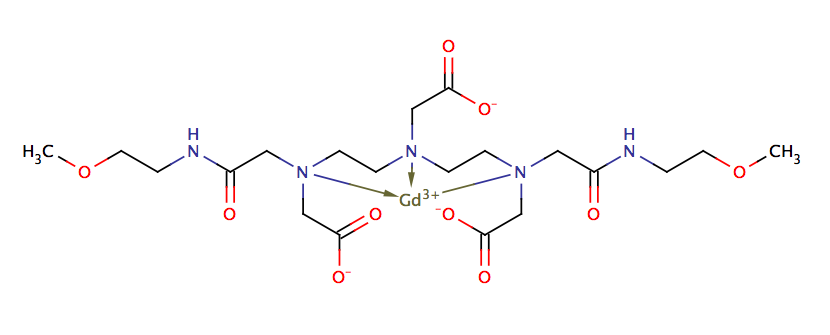
Structure
There is limited information regarding Gadoversetamide Structure in the drug label.
Pharmacodynamics
There is limited information regarding Gadoversetamide Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Gadoversetamide Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Gadoversetamide Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Gadoversetamide Clinical Studies in the drug label.
How Supplied
There is limited information regarding Gadoversetamide How Supplied in the drug label.
Storage
There is limited information regarding Gadoversetamide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Gadoversetamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Gadoversetamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Gadoversetamide Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Gadoversetamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Gadoversetamide Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Gadoversetamide Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.