Furan
Template:Chembox new Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Furan, also known as furane and furfuran, is a heterocyclic organic compound. It is typically derived by the thermal decomposition of pentose-containing materials, cellolosic solids especially pine-wood. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is toxic and may be carcinogenic. Catalytic hydrogenation (see redox) of furan with a palladium catalyst gives tetrahydrofuran.
Furan is aromatic because one of the lone pairs of electrons on the oxygen atom is delocalized into the ring, creating a 4n+2 aromatic system (see Hückel's rule) similar to benzene. Because of the aromaticity, the molecule is flat and lacks discrete double bonds. The other lone pair of electrons of the oxygen atom extends in the plane of the flat ring system. The sp2 hybridization is to allow one of the lone pairs of oxygen to reside in a p orbital and thus allow it to interact within the pi-system.
The name furan comes from the Latin furfur, which means bran.[1] The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limpricht in 1870, although he called it tetraphenol.[2][3]
Synthesis and isolation
- Furan can be obtained from furfural by oxidation and decarboxylation of the resulting furan-2-carboxylic acid, the furfural being derived by destructive distillation of corn cobs in the presence of sulfuric acid.[4]
- A classic furan organic synthesis is the Feist-Benary synthesis.
- One of the most simple synthesis methods for furans is the reaction of 1,4-diketones with phosphorus pentoxide (P2O5) in the Paal-Knorr Synthesis. It is interesting that the thiophene formation reaction of 1,4-diketones with Lawesson's reagent also forms furans as side products.
Reactions
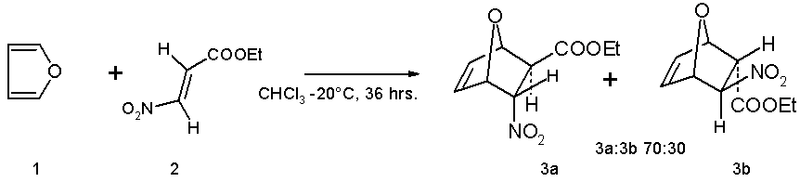
Due to its aromaticity, furan's behavior is quite dissimilar to that of the more typical heterocyclic ethers such as tetrahydrofuran. It is considerably more reactive than benzene in electrophilic substitution reactions. Furan serves as a diene in Diels-Alder reactions with electron-deficient dienophiles such as ethyl (E)-3-nitroacrylate.[5] The reaction product is a mixture of isomers with preference for the endo isomer:
Hydrogenation of furans affords sequentially dihydrofurans and tetrahydrofurans.
See also
- 2,5-Dimethylfuran, a furan derivative which may be a possible biofuel
- Tetrahydrofuran (THF), the fully hydrogenated analog of furan and a common solvent.
- Pyrrole, the nitrogen analog of furan.
- Thiophene, the sulfur analog of furan.
- Selenophene, the selenium analog of furan.
- Tellurophene, the tellurium analog of furan.
- Benzofuran, furan with a fused benzene ring.
- Dibenzofuran, a compound class similar to dibenzodioxins.
- Simple aromatic rings
- Furanose
References
- ↑ Alexander Senning. Elsevier's Dictionary of Chemoetymology. Elsevier, 2006. ISBN 0444522395.
- ↑ H. Limpricht (1870). "Ueber das Tetraphenol C4H4O". Berichte der deutschen chemischen Gesellschaft. 3 (1): 90–91. doi:10.1002/cber.18700030129.
- ↑ Ernest Harry Rodd. Chemistry of Carbon Compounds: A Modern Comprehensive Treatise. Elsevier, 1971.
- ↑ Wilson, W. C. "Furan" Organic Syntheses, Collected Volume 1, p.274 (1941). http://www.orgsyn.org/orgsyn/pdfs/CV1P0274.pdf
- ↑ Masesane I, Batsanov A, Howard J, Modal R, Steel P (2006). "The oxanorbornene approach to 3-hydroxy, 3,4-dihydroxy and 3,4,5-trihydroxy derivatives of 2-aminocyclohexanecarboxylic acid". Beilstein Journal of Organic Chemistry. 2 (9). doi:10.1186/1860-5397-2-9.
External links
de:Furan it:Furano lv:Furāns nl:Furaan sk:Furán fi:Furaani sv:Furan Template:Jb1 Template:WH Template:WS