Fragment antigen binding: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EJ}} + & -{{EH}} + & -{{Editor Join}} + & -{{Editor Help}} +)) |
m (Robot: Automated text replacement (-{{WikiDoc Cardiology Network Infobox}} +, -<references /> +{{reflist|2}}, -{{reflist}} +{{reflist|2}})) |
||
| Line 7: | Line 7: | ||
*[[Fc region]] | *[[Fc region]] | ||
== References == | == References == | ||
{{reflist}} | {{reflist|2}} | ||
[[Category:Immunology]] | [[Category:Immunology]] | ||
Latest revision as of 17:29, 4 September 2012
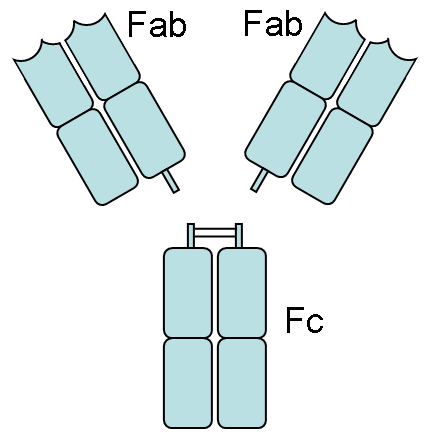
The fragment antigen binding (Fab fragment) is a region on an antibody which binds to antigens. It is composed of one constant and one variable domain of each of the heavy and the light chain. These domains shape the paratope—the antigen binding site—at the amino terminal end of the monomer. The two variable domains bind the epitope on their specific antigens.
In an experimental setting, Fc and Fab fragments can be generated in the laboratory. The enzyme papain can be used to cleave an immunoglobulin monomer into two Fab fragments and an Fc fragment. The enzyme pepsin cleaves below hinge region, so a F(ab')2 fragment and a Fc fragment is formed. The variable regions of the heavy and light chains can be fused together to form a single chain variable fragment (scFv), which is only half the size of the Fab fragment yet retains the original specificity of the parent immunoglobulin[1].
See also
References
- ↑ Janeway CA, Jr.; et al. (2001). Immunobiology (5th ed. ed.). Garland Publishing. (electronic full text via NCBI Bookshelf) ISBN 0-8153-3642-X.