Eprosartan: Difference between revisions
Joao Silva (talk | contribs) No edit summary |
Joao Silva (talk | contribs) No edit summary |
||
| Line 425: | Line 425: | ||
}} | }} | ||
|mechAction=(Description) | |mechAction=(Description) | ||
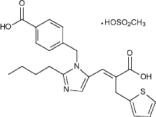
|structure= | |structure=* Eprosartan mesylate is a non-biphenyl non-tetrazole angiotensin II receptor (AT1) antagonist. A selective non-peptide molecule, eprosartan mesylate is chemically described as the monomethanesulfonate of (E )-2-butyl-1-(p-carboxybenzyl)-α-2-thienylmethylimid-azole-5-acrylic acid. | ||
Its empirical formula is C23H24N2O4S•CH4O3S and molecular weight is 520.625. Its structural formula is: | * Its empirical formula is C23H24N2O4S•CH4O3S and molecular weight is 520.625. Its structural formula is: | ||
[[File:Eprosartan structure.jpeg|500px|thumbnail|left|This image is provided by the National Library of Medicine.]] | [[File:Eprosartan structure.jpeg|500px|thumbnail|left|This image is provided by the National Library of Medicine.]] | ||
{{clr}} | {{clr}} | ||
Eprosartan mesylate is a white to off-white free-flowing crystalline powder that is insoluble in water, freely soluble in ethanol, and melts between 248°C and 250°C. | * Eprosartan mesylate is a white to off-white free-flowing crystalline powder that is insoluble in water, freely soluble in ethanol, and melts between 248°C and 250°C. | ||
* Eprosartan mesylate is available as aqueous film-coated tablets containing eprosartan mesylate equivalent to 400 mg or 600 mg eprosartan zwitterion (pink, oval, non-scored tablets or white, non-scored, capsule-shaped tablets, respectively). | |||
* Inactive Ingredients: | |||
:* The 400 mg tablet contains the following: croscarmellose sodium, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide. The 600 mg tablet contains crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide. | |||
|PD=(Description) | |PD=(Description) | ||
|PK=(Description) | |PK=(Description) | ||
Revision as of 21:28, 1 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: João André Alves Silva, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
Condition Name:
See full prescribing information for complete boxed warning.
|
Overview
Eprosartan is an angiotensin II receptor blocker that is FDA approved for the {{{indicationType}}} of hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include abdominal pain, myalgia, dizziness, upper respiratory infection and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
- Condition 1
- Condition 2
- Condition 3
- Condition 4
- Condition 5
Warnings
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
Condition Name:
See full prescribing information for complete boxed warning.
|
Conidition 1
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
(Description)
Labor and Delivery
(Description)
Nursing Mothers
(Description)
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Eprosartan
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
- Eprosartan mesylate is a non-biphenyl non-tetrazole angiotensin II receptor (AT1) antagonist. A selective non-peptide molecule, eprosartan mesylate is chemically described as the monomethanesulfonate of (E )-2-butyl-1-(p-carboxybenzyl)-α-2-thienylmethylimid-azole-5-acrylic acid.
- Its empirical formula is C23H24N2O4S•CH4O3S and molecular weight is 520.625. Its structural formula is:
- Eprosartan mesylate is a white to off-white free-flowing crystalline powder that is insoluble in water, freely soluble in ethanol, and melts between 248°C and 250°C.
- Eprosartan mesylate is available as aqueous film-coated tablets containing eprosartan mesylate equivalent to 400 mg or 600 mg eprosartan zwitterion (pink, oval, non-scored tablets or white, non-scored, capsule-shaped tablets, respectively).
- Inactive Ingredients:
- The 400 mg tablet contains the following: croscarmellose sodium, hypromellose, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide. The 600 mg tablet contains crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, polysorbate 80, pregelatinized starch, and titanium dioxide.
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
- TEVETEN® is available as aqueous film-coated tablets as follows:
- 400 mg pink, non-scored, oval tablets, debossed with “5044” on one side.
- NDC 0074–3025–11 (bottles of 100)
- 600 mg white, non-scored, capsule-shaped tablets, debossed with “5046” on one side.
- NDC 0074–3040–11 (bottles of 100)
Storage
Store at controlled room temperature 20 to 25°C (68 to 77°F) [see USP Controlled Room Temperature].
Images
Drug Images
{{#ask: Page Name::Eprosartan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Eprosartan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
- Some patients previously exposed to eprosartan showed signs of alcohol intolerance, however, it is not possible to confirm if eprosartan was directly involved in the adverse reaction.
Brand Names
Teveten®
Look-Alike Drug Names
N/A
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.