Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
Lactic acidosis and severe hepatomegaly with steatosis and Post treatment exacerbation of hepatitis B:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of ATRIPLA. ATRIPLA is not approved for the treatment of chronic hepatitis B virus (HBV) infection. Severe acute exacerbations of hepatitis B have been reported in patients coinfected with HBV and HIV-1 who have discontinued EMTRIVA or VIREAD, two of the components of ATRIPLA. Hepatic function should be monitored closely in these patients. If appropriate, initiation of anti-hepatitis B therapy may be warranted. |
Overview
Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate is an Anti-HIV Agent that is FDA approved for the treatment of HIV-1 infection. There is a Black Box Warning for this drug as shown here. Common adverse reactions include diarrhea, nausea, fatigue, headache, dizziness, depression, insomnia, abnormal dreams, and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in pediatric patients.
Contraindications
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Contraindications in the drug label.
Warnings
|
WARNING: LACTIC ACIDOSIS/SEVERE HEPATOMEGALY WITH STEATOSIS and POST TREATMENT EXACERBATION OF HEPATITIS B
See full prescribing information for complete Boxed Warning.
Lactic acidosis and severe hepatomegaly with steatosis and Post treatment exacerbation of hepatitis B:
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate, a component of ATRIPLA. ATRIPLA is not approved for the treatment of chronic hepatitis B virus (HBV) infection. Severe acute exacerbations of hepatitis B have been reported in patients coinfected with HBV and HIV-1 who have discontinued EMTRIVA or VIREAD, two of the components of ATRIPLA. Hepatic function should be monitored closely in these patients. If appropriate, initiation of anti-hepatitis B therapy may be warranted. |
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in geriatric settings.
Gender
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Administration in the drug label.
Monitoring
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate and IV administrations.
Overdosage
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Pharmacology in the drug label.
Mechanism of Action
ATRIPLA is a fixed-dose combination of antiviral drugs efavirenz, emtricitabine and tenofovir disoproxil fumarate
Structure
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Structure in the drug label.
Pharmacodynamics
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Pharmacodynamics in the drug label.
Pharmacokinetics
ATRIPLA: One ATRIPLA tablet is bioequivalent to one SUSTIVA tablet (600 mg) plus one EMTRIVA capsule (200 mg) plus one VIREAD tablet (300 mg) following single-dose administration to fasting healthy subjects (N=45).
Efavirenz: In HIV-1 infected subjects time-to-peak plasma concentrations were approximately 3–5 hours and steady-state plasma concentrations were reached in 6–10 days. In 35 HIV-1 infected subjects receiving efavirenz 600 mg once daily, steady-state Cmax was 12.9 ± 3.7 µM (mean ± SD), Cmin was 5.6 ± 3.2 µM, and AUC was 184 ± 73 µM•hr. Efavirenz is highly bound (approximately 99.5–99.75%) to human plasma proteins, predominantly albumin. Following administration of 14C-labeled efavirenz, 14–34% of the dose was recovered in the urine (mostly as metabolites) and 16–61% was recovered in feces (mostly as parent drug). In vitro studies suggest CYP3A and CYP2B6 are the major isozymes responsible for efavirenz metabolism. Efavirenz has been shown to induce CYP enzymes, resulting in induction of its own metabolism. Efavirenz has a terminal half-life of 52–76 hours after single doses and 40–55 hours after multiple doses.
Emtricitabine: Following oral administration, emtricitabine is rapidly absorbed, with peak plasma concentrations occurring at 1–2 hours post-dose. Following multiple dose oral administration of emtricitabine to 20 HIV-1 infected subjects, the steady-state plasma emtricitabine Cmax was 1.8 ± 0.7 µg/mL (mean ± SD) and the AUC over a 24-hour dosing interval was 10.0 ± 3.1 µg•hr/mL. The mean steady-state plasma trough concentration at 24 hours post-dose was 0.09 µg/mL. The mean absolute bioavailability of emtricitabine was 93%. Less than 4% of emtricitabine binds to human plasma proteins in vitro and the binding is independent of concentration over the range of 0.02–200 µg/mL. Following administration of radiolabelled emtricitabine, approximately 86% is recovered in the urine and 13% is recovered as metabolites. The metabolites of emtricitabine include 3'-sulfoxide diastereomers and their glucuronic acid conjugate. Emtricitabine is eliminated by a combination of glomerular filtration and active tubular secretion with a renal clearance in adults with normal renal function of 213 ± 89 mL/min (mean ± SD). Following a single oral dose, the plasma emtricitabine half-life is approximately 10 hours.
Tenofovir Disoproxil Fumarate: Following oral administration of a single 300 mg dose of tenofovir DF to HIV-1 infected subjects in the fasted state, maximum serum concentrations (Cmax) were achieved in 1.0 ± 0.4 hrs (mean ± SD) and Cmax and AUC values were 296 ± 90 ng/mL and 2287 ± 685 ng•hr/mL, respectively. The oral bioavailability of tenofovir from tenofovir DF in fasted subjects is approximately 25%. Less than 0.7% of tenofovir binds to human plasma proteins in vitro and the binding is independent of concentration over the range of 0.01–25 µg/mL. Approximately 70–80% of the intravenous dose of tenofovir is recovered as unchanged drug in the urine. Tenofovir is eliminated by a combination of glomerular filtration and active tubular secretion with a renal clearance in adults with normal renal function of 243 ± 33 mL/min (mean ± SD). Following a single oral dose, the terminal elimination half-life of tenofovir is approximately 17 hours.
Effects of Food on Oral Absorption
ATRIPLA has not been evaluated in the presence of food. Administration of efavirenz tablets with a high fat meal increased the mean AUC and Cmax of efavirenz by 28% and 79%, respectively, compared to administration in the fasted state. Compared to fasted administration, dosing of tenofovir DF and emtricitabine in combination with either a high fat meal or a light meal increased the mean AUC and Cmax of tenofovir by 35% and 15%, respectively, without affecting emtricitabine exposures [See Dosage and Administration (2) and Patient Counseling Information (17.7)].
Special Populations
Race
Efavirenz: The pharmacokinetics of efavirenz in HIV-1 infected subjects appear to be similar among the racial groups studied.
Emtricitabine: No pharmacokinetic differences due to race have been identified following the administration of emtricitabine.
Tenofovir Disoproxil Fumarate: There were insufficient numbers from racial and ethnic groups other than Caucasian to adequately determine potential pharmacokinetic differences among these populations following the administration of tenofovir DF.
Gender
Efavirenz, Emtricitabine, and Tenofovir Disoproxil Fumarate: Efavirenz, emtricitabine, and tenofovir pharmacokinetics are similar in male and female subjects.
Pediatric Patients
ATRIPLA should only be administered to pediatric patients 12 years of age and weighing greater than or equal to 40 kg (greater than or equal to 88 lb).
Efavirenz: In an open-label trial in NRTI-experienced pediatric subjects (mean age 8 years, range 3–16), the pharmacokinetics of efavirenz in pediatric subjects were similar to the pharmacokinetics in adults who received a 600 mg daily dose of efavirenz. In 48 pediatric subjects, receiving the equivalent of a 600 mg dose of efavirenz, mean (± SD) steady-state Cmax was 14.2 ± 5.8 µM, steady-state Cmin was 5.6 ± 4.1 µM, and AUC was 218 ± 104 µM•hr.
Emtricitabine: The pharmacokinetics of emtricitabine at steady state were determined in 27 HIV-1-infected pediatric subjects 13 to 17 years of age receiving a daily dose of 6 mg/kg up to a maximum dose of 240 mg oral solution or a 200 mg capsule; 26 of 27 subjects in this age group received the 200 mg EMTRIVA capsule. Mean (± SD) Cmax and AUC were 2.7 ± 0.9 µg/mL and 12.6 ± 5.4 µg•hr/mL, respectively. Exposures achieved in pediatric subjects 12 to less than 18 years of age were similar to those achieved in adults receiving a once daily dose of 200 mg.
Tenofovir Disoproxil Fumarate: Steady-state pharmacokinetics of tenofovir were evaluated in 8 HIV-1 infected pediatric subjects (12 to less than 18 years). Mean (± SD) Cmax and AUCtau are 0.38 ± 0.13 µg/mL and 3.39 ± 1.22 µg•hr/mL, respectively. Tenofovir exposure achieved in these pediatric subjects receiving oral daily doses of VIREAD 300 mg was similar to exposures achieved in adults receiving once-daily doses of VIREAD 300 mg.
Geriatric Patients
Pharmacokinetics of efavirenz, emtricitabine and tenofovir have not been fully evaluated in the elderly (65 years of age and older) [See Use in Specific Populations (8.5)].
Patients with Impaired Renal Function
Efavirenz: The pharmacokinetics of efavirenz have not been studied in subjects with renal insufficiency; however, less than 1% of efavirenz is excreted unchanged in the urine, so the impact of renal impairment on efavirenz elimination should be minimal.
Emtricitabine and Tenofovir Disoproxil Fumarate: The pharmacokinetics of emtricitabine and tenofovir DF are altered in subjects with renal impairment. In subjects with creatinine clearance below 50 mL/min, Cmax and AUC0–∞ of emtricitabine and tenofovir were increased [See Warnings and Precautions (5.7)].
Patients with Hepatic Impairment
Efavirenz: A multiple-dose trial showed no significant effect on efavirenz pharmacokinetics in subjects with mild hepatic impairment (Child-Pugh Class A) compared with controls. There were insufficient data to determine whether moderate or severe hepatic impairment (Child-Pugh Class B or C) affects efavirenz pharmacokinetics [See Warnings and Precautions (5.10) and Use in Specific Populations (8.6)].
Emtricitabine: The pharmacokinetics of emtricitabine have not been studied in subjects with hepatic impairment; however, emtricitabine is not significantly metabolized by liver enzymes, so the impact of liver impairment should be limited.
Tenofovir Disoproxil Fumarate: The pharmacokinetics of tenofovir following a 300 mg dose of tenofovir DF have been studied in non-HIV infected subjects with moderate to severe hepatic impairment. There were no substantial alterations in tenofovir pharmacokinetics in subjects with hepatic impairment compared with unimpaired subjects.
Assessment of Drug Interactions
The drug interaction trials described were conducted with efavirenz, emtricitabine, or tenofovir DF as individual agents; no drug interaction trials have been conducted using ATRIPLA.
Efavirenz: The steady-state pharmacokinetics of efavirenz and tenofovir were unaffected when efavirenz and tenofovir DF were administered together versus each agent dosed alone. Specific drug interaction trials have not been performed with efavirenz and NRTIs other than tenofovir, lamivudine, and zidovudine. Clinically significant interactions would not be expected based on NRTIs elimination pathways.
Efavirenz has been shown in vivo to cause hepatic enzyme induction, thus increasing the biotransformation of some drugs metabolized by CYP3A and CYP2B6. In vitro studies have shown that efavirenz inhibited CYP isozymes 2C9, 2C19, and 3A4 with Ki values (8.5–17 µM) in the range of observed efavirenz plasma concentrations. In in vitro studies, efavirenz did not inhibit CYP2E1 and inhibited CYP2D6 and CYP1A2 (Ki values 82–160 µM) only at concentrations well above those achieved clinically. Coadministration of efavirenz with drugs primarily metabolized by 2C9, 2C19, and 3A4 isozymes may result in altered plasma concentrations of the coadministered drug. Drugs which induce CYP3A activity would be expected to increase the clearance of efavirenz resulting in lowered plasma concentrations.
Drug interaction trials were performed with efavirenz and other drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interaction. There was no clinically significant interaction observed between efavirenz and zidovudine, lamivudine, azithromycin, fluconazole, lorazepam, cetirizine, or paroxetine. Single doses of famotidine or an aluminum and magnesium antacid with simethicone had no effects on efavirenz exposures. The effects of coadministration of efavirenz on Cmax, AUC, and Cmin are summarized in Table 5 (effect of other drugs on efavirenz) and Table 6 (effect of efavirenz on other drugs). For information regarding clinical recommendations see Drug Interactions (7).
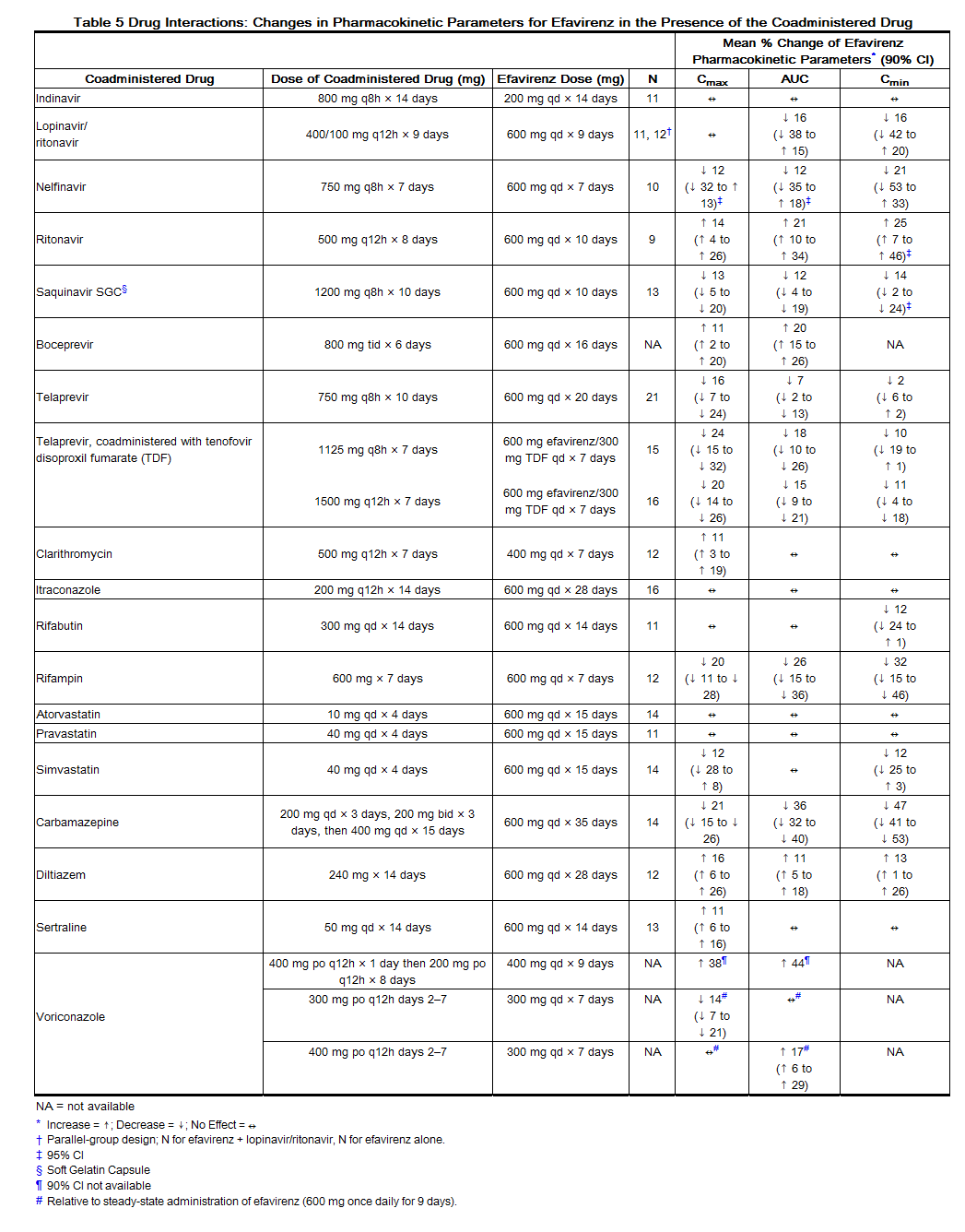
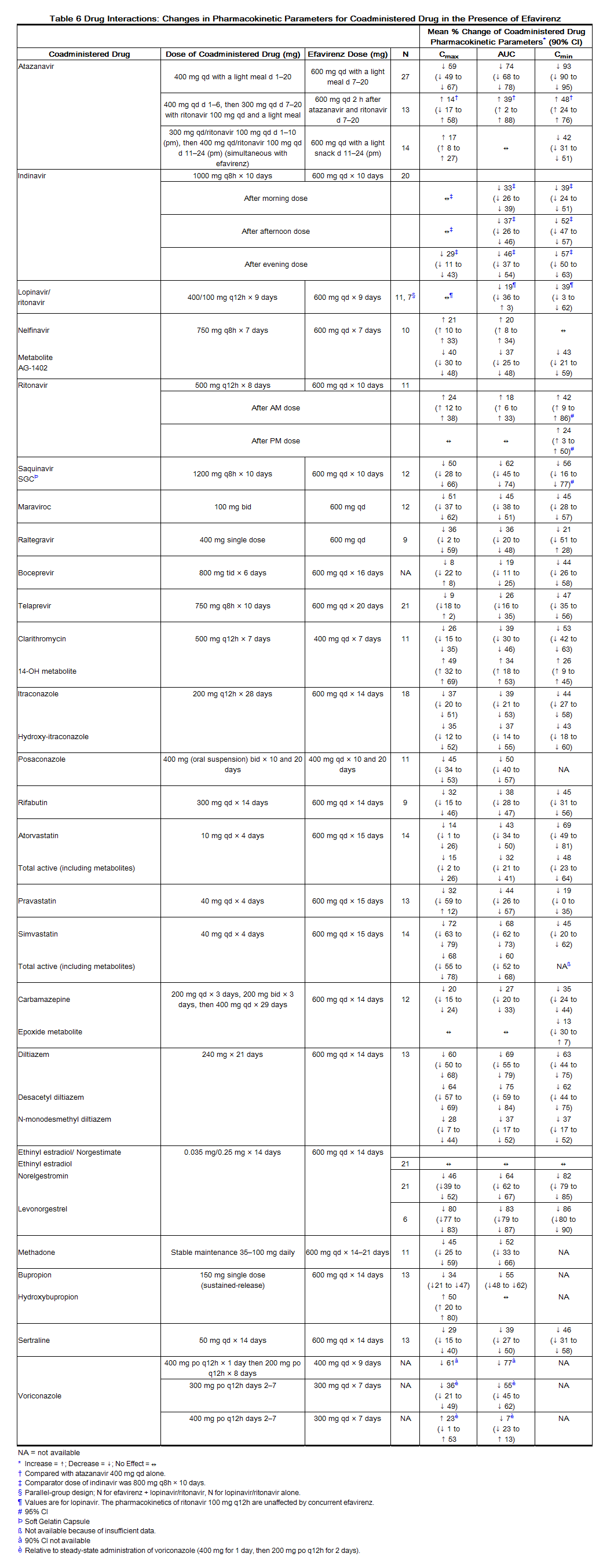
Emtricitabine and Tenofovir Disoproxil Fumarate: The steady-state pharmacokinetics of emtricitabine and tenofovir were unaffected when emtricitabine and tenofovir DF were administered together versus each agent dosed alone.
In vitro and clinical pharmacokinetic drug-drug interaction studies have shown that the potential for CYP mediated interactions involving emtricitabine and tenofovir with other medicinal products is low.
Emtricitabine and tenofovir are primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion. No drug-drug interactions due to competition for renal excretion have been observed; however, coadministration of emtricitabine and tenofovir DF with drugs that are eliminated by active tubular secretion may increase concentrations of emtricitabine, tenofovir, and/or the coadministered drug.
Drugs that decrease renal function may increase concentrations of emtricitabine and/or tenofovir.
No clinically significant drug interactions have been observed between emtricitabine and famciclovir, indinavir, stavudine, tenofovir DF and zidovudine. Similarly, no clinically significant drug interactions have been observed between tenofovir DF and abacavir, efavirenz, emtricitabine, entecavir, indinavir, lamivudine, lopinavir/ritonavir, methadone, nelfinavir, oral contraceptives, ribavirin, saquinavir/ritonavir or tacrolimus in trials conducted in healthy volunteers.
Following multiple dosing to HIV-negative subjects receiving either chronic methadone maintenance therapy, oral contraceptives, or single doses of ribavirin, steady-state tenofovir pharmacokinetics were similar to those observed in previous trials, indicating a lack of clinically significant drug interactions between these agents and tenofovir DF. The effects of coadministered drugs on the Cmax, AUC, and Cmin of tenofovir are shown in Table 7. The effects of coadministration of tenofovir DF on Cmax, AUC, and Cmin of coadministered drugs are shown in Table 8.
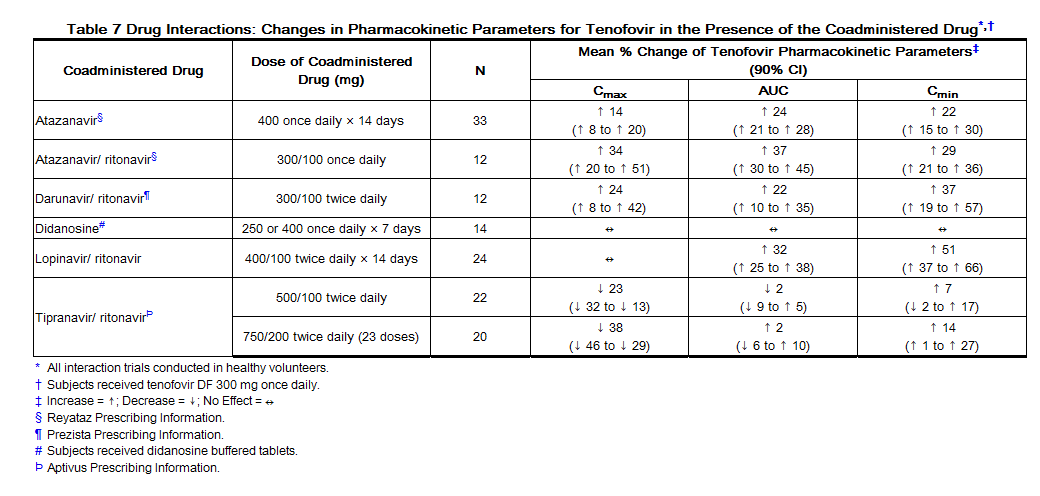
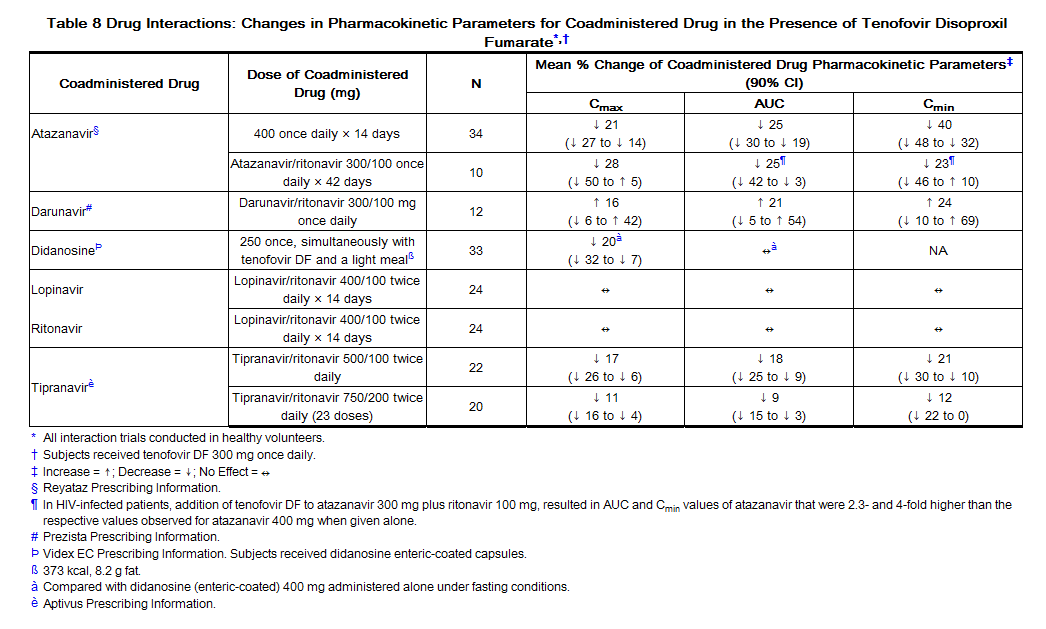
Coadministration of tenofovir DF with didanosine results in changes in the pharmacokinetics of didanosine that may be of clinical significance. Concomitant dosing of tenofovir DF with didanosine enteric-coated capsules significantly increases the Cmax and AUC of didanosine. When didanosine 250 mg enteric-coated capsules were administered with tenofovir DF, systemic exposures of didanosine were similar to those seen with the 400 mg enteric-coated capsules alone under fasted conditions. The mechanism of this interaction is unknown [for didanosine dosing adjustment recommendations see Drug Interactions (7.3), Table 4].
Nonclinical Toxicology
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Clinical Studies in the drug label.
How Supplied
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate How Supplied in the drug label.
Storage
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Efavirenz, Emtricitabine, and Tenofovir disoproxil fumarate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.