Desogestrel and Ethinyl Estradiol: Difference between revisions
No edit summary |
No edit summary |
||
| Line 32: | Line 32: | ||
|warnings='''Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, combination oral contraceptives, including Apri®, should not be used by women who are over 35 years of age and smoke.''' | |warnings='''Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, combination oral contraceptives, including Apri®, should not be used by women who are over 35 years of age and smoke.''' | ||
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity and diabetes. | The use of [[oral contraceptives]] is associated with increased risks of several serious conditions including [[myocardial infarction]], [[thromboembolism]], [[stroke]], [[hepatic neoplasia]], and [[gallbladder disease]], although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as [[hypertension]], [[hyperlipidemias]], [[obesity]] and [[diabetes]]. | ||
Practitioners prescribing oral contraceptives should be familiar with the following information relating to these risks. | Practitioners prescribing oral contraceptives should be familiar with the following information relating to these risks. | ||
The information contained in this package insert is principally based on studies carried out in patients who used oral contraceptives with formulations of higher doses of estrogens and progestogens than those in common use today. The effect of long term use of the oral contraceptives with formulations of lower doses of both estrogens and progestogens remains to be determined. | The information contained in this package insert is principally based on studies carried out in patients who used oral contraceptives with formulations of higher doses of estrogens and [[progestogens]] than those in common use today. The effect of long term use of the [[oral contraceptives]] with formulations of lower doses of both [[estrogens]] and [[progestogens]] remains to be determined. | ||
Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among nonusers. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between oral contraceptive users and nonusers. The attributable risk does provide information about the actual occurrence of a disease in the population (Adapted from refs. 2 and 3 with the author’s permission). For further information, the reader is referred to a text on epidemiological methods. | Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among [[oral contraceptive]] users to that among nonusers. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between [[oral contraceptive]] users and nonusers. The attributable risk does provide information about the actual occurrence of a disease in the population (Adapted from refs. 2 and 3 with the author’s permission). For further information, the reader is referred to a text on epidemiological methods. | ||
====Thromboembolic Disorder and Other Vascular Problems==== | ====Thromboembolic Disorder and Other Vascular Problems==== | ||
=====Thromboembolism===== | =====Thromboembolism===== | ||
An increased risk of thromboembolic and thrombotic disease associated with the use of oral contraceptives is well established. Case control studies have found the relative risk of users compared to nonusers to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease 2,3,19 to 24. Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization 25. The risk of thromboembolic disease associated with oral contraceptives is not related to length of use and disappears after pill use is | An increased risk of [[thromboembolic]] and [[thrombotic disease]] associated with the use of [[oral contraceptives]] is well established. Case control studies have found the relative risk of users compared to nonusers to be 3 for the first episode of superficial [[venous thrombosis]], 4 to 11 for deep [[vein thrombosis]] or [[pulmonary embolism]], and 1.5 to 6 for women with predisposing conditions for venous [[thromboembolic disease]] 2,3,19 to 24. Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization 25. The risk of [[thromboembolic disease]] associated with [[oral contraceptives]] is not related to length of use and disappears after pill use is stopped. Several epidemiologic studies indicate that third generation [[oral contraceptives]], including those containing desogestrel, are associated with a higher risk of [[venous thromboembolism]] than certain second generation [[oral contraceptives]]. In general, these studies indicate an approximate 2-fold increased risk, which corresponds to an additional 1 to 2 cases of [[venous thromboembolism]] per 10,000 women-years of use. However, data from additional studies have not shown this 2-fold increase in risk. | ||
A two- to four-fold increase in relative risk of post-operative thromboembolic complications has been reported with the use of oral | A two- to four-fold increase in relative risk of post-operative [[thromboembolic]] complications has been reported with the use of [[oral contraceptives]]. The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions. If feasible, [[oral contraceptives]] should be discontinued at least four weeks prior to and for two weeks after elective surgery of a type associated with an increase in risk of [[thromboembolism]] and during and following prolonged immobilization. Since the immediate postpartum period is also associated with an increased risk of [[thromboembolism]], [[oral contraceptives]] should be started no earlier than four weeks after delivery in women who elect not to breastfeed. | ||
=====Myocardial Infarction===== | =====Myocardial Infarction===== | ||
| Line 52: | Line 52: | ||
Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older with smoking accounting for the majority of excess cases11. Mortality rates associated with circulatory disease have been shown to increase substantially in smokers, especially in those 35 years of age and older and in nonsmokers over the age of 40 among women who use oral contraceptives (see Figure 1). | Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older with smoking accounting for the majority of excess cases11. Mortality rates associated with circulatory disease have been shown to increase substantially in smokers, especially in those 35 years of age and older and in nonsmokers over the age of 40 among women who use oral contraceptives (see Figure 1). | ||
[[file:Warning1 DE.png|none|350px]] | |||
Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age and obesity13. In particular, some progestogens are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism14 to 18. Oral contraceptives have been shown to increase blood pressure among users (see section 9 in WARNINGS). Similar effects on risk factors have been associated with an increased risk of heart disease. Oral contraceptives must be used with caution in women with cardiovascular disease risk factors. | Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age and obesity13. In particular, some progestogens are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism14 to 18. Oral contraceptives have been shown to increase blood pressure among users (see section 9 in WARNINGS). Similar effects on risk factors have been associated with an increased risk of heart disease. Oral contraceptives must be used with caution in women with cardiovascular disease risk factors. | ||
Revision as of 21:03, 26 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Desogestrel and Ethinyl Estradiol is an oral contraceptive that is FDA approved for the prophylaxis of unplanned pregnancy in women who elect to use oral contraceptives as a method of contraception. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Desogestrel and Ethinyl Estradiol FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Desogestrel and Ethinyl Estradiol FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Desogestrel and Ethinyl Estradiol in pediatric patients.
Contraindications
Oral contraceptives should not be used in women who currently have the following conditions:
- Thrombophlebitis or thromboembolic disorders
- A past history of deep vein thrombophlebitis or thromboembolic disorders
- Known thrombophilic conditions
- Cerebral vascular disease or coronary artery disease (current or history)
- Valvular heart disease with complications
- Persistent blood pressure values of > 160 mm Hg systolic or > 100 mg Hg diastolic
- Diabetes with vascular involvement
- Headaches with focal neurological symptoms
- Major surgery with prolonged immobilization
- Known or suspected carcinoma of the breast or personal history of breast cancer
- Endometrial carcinoma or other known or suspected estrogen-dependent neoplasia
- Undiagnosed abnormal genital bleeding
- Cholestatic jaundice of pregnancy or jaundice with prior pill use
- Acute or chronic hepatocellular disease with abnormal liver function
- Hepatic adenomas or carcinomas
- Known or suspected pregnancy
- Hypersensitivity to any component of this product
Warnings
Cigarette smoking increases the risk of serious cardiovascular events from combination oral contraceptive use. This risk increases with age, particularly in women over 35 years of age, and with the number of cigarettes smoked. For this reason, combination oral contraceptives, including Apri®, should not be used by women who are over 35 years of age and smoke.
The use of oral contraceptives is associated with increased risks of several serious conditions including myocardial infarction, thromboembolism, stroke, hepatic neoplasia, and gallbladder disease, although the risk of serious morbidity or mortality is very small in healthy women without underlying risk factors. The risk of morbidity and mortality increases significantly in the presence of other underlying risk factors such as hypertension, hyperlipidemias, obesity and diabetes.
Practitioners prescribing oral contraceptives should be familiar with the following information relating to these risks.
The information contained in this package insert is principally based on studies carried out in patients who used oral contraceptives with formulations of higher doses of estrogens and progestogens than those in common use today. The effect of long term use of the oral contraceptives with formulations of lower doses of both estrogens and progestogens remains to be determined.
Throughout this labeling, epidemiological studies reported are of two types: retrospective or case control studies and prospective or cohort studies. Case control studies provide a measure of the relative risk of a disease, namely, a ratio of the incidence of a disease among oral contraceptive users to that among nonusers. The relative risk does not provide information on the actual clinical occurrence of a disease. Cohort studies provide a measure of attributable risk, which is the difference in the incidence of disease between oral contraceptive users and nonusers. The attributable risk does provide information about the actual occurrence of a disease in the population (Adapted from refs. 2 and 3 with the author’s permission). For further information, the reader is referred to a text on epidemiological methods.
Thromboembolic Disorder and Other Vascular Problems
Thromboembolism
An increased risk of thromboembolic and thrombotic disease associated with the use of oral contraceptives is well established. Case control studies have found the relative risk of users compared to nonusers to be 3 for the first episode of superficial venous thrombosis, 4 to 11 for deep vein thrombosis or pulmonary embolism, and 1.5 to 6 for women with predisposing conditions for venous thromboembolic disease 2,3,19 to 24. Cohort studies have shown the relative risk to be somewhat lower, about 3 for new cases and about 4.5 for new cases requiring hospitalization 25. The risk of thromboembolic disease associated with oral contraceptives is not related to length of use and disappears after pill use is stopped. Several epidemiologic studies indicate that third generation oral contraceptives, including those containing desogestrel, are associated with a higher risk of venous thromboembolism than certain second generation oral contraceptives. In general, these studies indicate an approximate 2-fold increased risk, which corresponds to an additional 1 to 2 cases of venous thromboembolism per 10,000 women-years of use. However, data from additional studies have not shown this 2-fold increase in risk.
A two- to four-fold increase in relative risk of post-operative thromboembolic complications has been reported with the use of oral contraceptives. The relative risk of venous thrombosis in women who have predisposing conditions is twice that of women without such medical conditions. If feasible, oral contraceptives should be discontinued at least four weeks prior to and for two weeks after elective surgery of a type associated with an increase in risk of thromboembolism and during and following prolonged immobilization. Since the immediate postpartum period is also associated with an increased risk of thromboembolism, oral contraceptives should be started no earlier than four weeks after delivery in women who elect not to breastfeed.
Myocardial Infarction
An increased risk of myocardial infarction has been attributed to oral contraceptive use. This risk is primarily in smokers or women with other underlying risk factors for coronary artery disease such as hypertension, hypercholesterolemia, morbid obesity, and diabetes. The relative risk of heart attack for current oral contraceptive users has been estimated to be two to six4 to 10. The risk is very low in women under the age of 30.
Smoking in combination with oral contraceptive use has been shown to contribute substantially to the incidence of myocardial infarctions in women in their mid-thirties or older with smoking accounting for the majority of excess cases11. Mortality rates associated with circulatory disease have been shown to increase substantially in smokers, especially in those 35 years of age and older and in nonsmokers over the age of 40 among women who use oral contraceptives (see Figure 1).
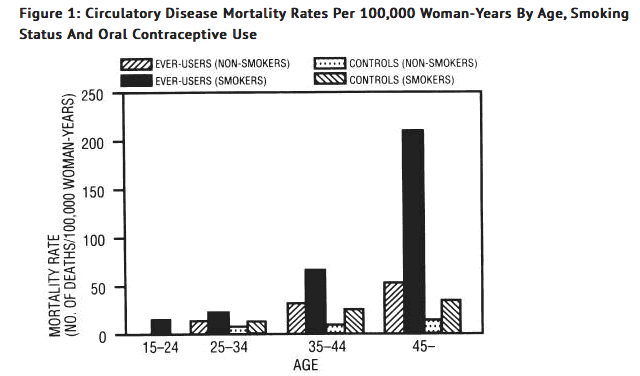
Oral contraceptives may compound the effects of well-known risk factors, such as hypertension, diabetes, hyperlipidemias, age and obesity13. In particular, some progestogens are known to decrease HDL cholesterol and cause glucose intolerance, while estrogens may create a state of hyperinsulinism14 to 18. Oral contraceptives have been shown to increase blood pressure among users (see section 9 in WARNINGS). Similar effects on risk factors have been associated with an increased risk of heart disease. Oral contraceptives must be used with caution in women with cardiovascular disease risk factors.
There is some evidence that the risk of myocardial infarction associated with oral contraceptives is lower when the progestogen has minimal androgenic activity than when the activity is greater. Receptor binding and animal studies have shown that desogestrel or its active metabolite has minimal androgenic activity (see CLINICAL PHARMACOLOGY), although these findings have not been confirmed in adequate and well-controlled clinical trials.
Cerebrovascular Diseases
Oral contraceptives have been shown to increase both the relative and attributable risks of cerebrovascular events (thrombotic and hemorrhagic strokes), although, in general, the risk is greatest among older (> 35 years), hypertensive women who also smoke. Hypertension was found to be a risk factor for both users and nonusers, for both types of strokes, and smoking interacted to increase the risk of stroke 27 to 29.
In a large study, the relative risk of thrombotic strokes has been shown to range from 3 for normotensive users to 14 for users with severe hypertension30. The relative risk of hemorrhagic stroke is reported to be 1.2 for non-smokers who used oral contraceptives, 2.6 for smokers who did not use oral contraceptives, 7.6 for smokers who used oral contraceptives, 1.8 for normotensive users and 25.7 for users with severe hypertension30. The attributable risk is also greater in older women3.
Dose-Related Risk of Vascular Disease from Oral Contraceptives
A positive association has been observed between the amount of estrogen and progestogen in oral contraceptives and the risk of vascular disease31 to 33. A decline in serum high density lipoproteins (HDL) has been reported with many progestational agents14 to 16. A decline in serum high density lipoproteins has been associated with an increased incidence of ischemic heart disease. Because estrogens increase HDL cholesterol, the net effect of an oral contraceptive depends on a balance achieved between doses of estrogen and progestogen and the nature and absolute amount of progestogens used in the contraceptives. The amount of both hormones should be considered in the choice of an oral contraceptive.
Minimizing exposure to estrogen and progestogen is in keeping with good principles of therapeutics. For any particular estrogen/progestogen combination, the dosage regimen prescribed should be one which contains the least amount of estrogen and progestogen that is compatible with a low failure rate and the needs of the individual patient. New acceptors of oral contraceptive agents should be started on preparations containing the lowest estrogen content which is judged appropriate for the individual patient.
Persistence of Risk of Vascular Disease
There are two studies which have shown persistence of risk of vascular disease for ever-users of oral contraceptives. In a study in the United States, the risk of developing myocardial infarction after discontinuing oral contraceptives persists for at least 9 years for women 40 to 49 years old who had used oral contraceptives for five or more years, but this increased risk was not demonstrated in other age groups8. In another study in Great Britain, the risk of developing cerebrovascular disease persisted for at least 6 years after discontinuation of oral contraceptives, although excess risk was very small34. However, both studies were performed with oral contraceptive formulations containing 0.050 mg or higher of estrogens.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Desogestrel and Ethinyl Estradiol Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Desogestrel and Ethinyl Estradiol Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Desogestrel and Ethinyl Estradiol Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Desogestrel and Ethinyl Estradiol in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Desogestrel and Ethinyl Estradiol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Desogestrel and Ethinyl Estradiol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in geriatric settings.
Gender
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Desogestrel and Ethinyl Estradiol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Desogestrel and Ethinyl Estradiol in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Desogestrel and Ethinyl Estradiol Administration in the drug label.
Monitoring
There is limited information regarding Desogestrel and Ethinyl Estradiol Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Desogestrel and Ethinyl Estradiol and IV administrations.
Overdosage
There is limited information regarding Desogestrel and Ethinyl Estradiol overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Desogestrel and Ethinyl Estradiol Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Desogestrel and Ethinyl Estradiol Mechanism of Action in the drug label.
Structure
There is limited information regarding Desogestrel and Ethinyl Estradiol Structure in the drug label.
Pharmacodynamics
There is limited information regarding Desogestrel and Ethinyl Estradiol Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Desogestrel and Ethinyl Estradiol Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Desogestrel and Ethinyl Estradiol Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Desogestrel and Ethinyl Estradiol Clinical Studies in the drug label.
How Supplied
There is limited information regarding Desogestrel and Ethinyl Estradiol How Supplied in the drug label.
Storage
There is limited information regarding Desogestrel and Ethinyl Estradiol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Desogestrel and Ethinyl Estradiol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Desogestrel and Ethinyl Estradiol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Desogestrel and Ethinyl Estradiol Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Desogestrel and Ethinyl Estradiol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Desogestrel and Ethinyl Estradiol Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Desogestrel and Ethinyl Estradiol Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.