Cystic fibrosis: Difference between revisions
| Line 63: | Line 63: | ||
One way in which infection has spread is by passage between different individuals with CF.<ref>Tummler B, Koopmann U, Grothues D, Weissbrodt H, Steinkamp G, von der Hardt H. ''Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients.'' J Clin Microbiol. 1991 Jun;29(6):1265–7. PMID 1907611</ref> In the past, people with CF often participated in summer "CF Camps" and other recreational gatherings.<ref>Centers for Disease Control and Prevention (CDC). ''Pseudomonas cepacia at summer camps for persons with cystic fibrosis.'' MMWR Morb Mortal Wkly Rep. 1993 Jun 18;42(23):456-9. PMID 7684813</ref><ref>Pegues DA, Carson LA, Tablan OC, FitzSimmons SC, Roman SB, Miller JM, Jarvis WR.''Acquisition of Pseudomonas cepacia at summer camps for patients with cystic fibrosis. Summer Camp Study Group.'' J Pediatr. 1994 May;124(5 Pt 1):694–702. PMID 7513755</ref> Hospitals grouped patients with CF into common areas and routine equipment (such as [[nebulizer]]s)<ref>Pankhurst CL, Philpott-Howard J. ''The environmental risk factors associated with medical and dental equipment in the transmission of Burkholderia (Pseudomonas) cepacia in cystic fibrosis patients.'' J Hosp Infect. 1996 Apr;32(4):249-55. PMID 8744509</ref> was not sterilized between individual patients.<ref>Jones AM, Govan JR, Doherty CJ, Dodd ME, Isalska BJ, Stanbridge TN, Webb AK. ''Identification of airborne dissemination of epidemic multiresistant strains of Pseudomonas aeruginosa at a CF centre during a cross infection outbreak.'' Thorax. 2003 Jun;58(6):525-7. PMID 12775867</ref> This led to transmission of more dangerous strains of bacteria among groups of patients. As a result, individuals with CF are routinely isolated from one another in the healthcare setting and healthcare providers are encouraged to wear gowns and gloves when examining patients with CF in order to limit the spread of virulent bacterial strains.<ref>Hoiby N. ''Isolation and treatment of cystic fibrosis patients with lung infections caused by Pseudomonas (Burkholderia) cepacia and multiresistant Pseudomonas aeruginosa.'' Neth J Med. 1995 Jun;46(6):280-7. PMID 7643943</ref> Often, patients with particularly damaging bacteria will attend clinics on different days and in different buildings than those without these infections. | One way in which infection has spread is by passage between different individuals with CF.<ref>Tummler B, Koopmann U, Grothues D, Weissbrodt H, Steinkamp G, von der Hardt H. ''Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients.'' J Clin Microbiol. 1991 Jun;29(6):1265–7. PMID 1907611</ref> In the past, people with CF often participated in summer "CF Camps" and other recreational gatherings.<ref>Centers for Disease Control and Prevention (CDC). ''Pseudomonas cepacia at summer camps for persons with cystic fibrosis.'' MMWR Morb Mortal Wkly Rep. 1993 Jun 18;42(23):456-9. PMID 7684813</ref><ref>Pegues DA, Carson LA, Tablan OC, FitzSimmons SC, Roman SB, Miller JM, Jarvis WR.''Acquisition of Pseudomonas cepacia at summer camps for patients with cystic fibrosis. Summer Camp Study Group.'' J Pediatr. 1994 May;124(5 Pt 1):694–702. PMID 7513755</ref> Hospitals grouped patients with CF into common areas and routine equipment (such as [[nebulizer]]s)<ref>Pankhurst CL, Philpott-Howard J. ''The environmental risk factors associated with medical and dental equipment in the transmission of Burkholderia (Pseudomonas) cepacia in cystic fibrosis patients.'' J Hosp Infect. 1996 Apr;32(4):249-55. PMID 8744509</ref> was not sterilized between individual patients.<ref>Jones AM, Govan JR, Doherty CJ, Dodd ME, Isalska BJ, Stanbridge TN, Webb AK. ''Identification of airborne dissemination of epidemic multiresistant strains of Pseudomonas aeruginosa at a CF centre during a cross infection outbreak.'' Thorax. 2003 Jun;58(6):525-7. PMID 12775867</ref> This led to transmission of more dangerous strains of bacteria among groups of patients. As a result, individuals with CF are routinely isolated from one another in the healthcare setting and healthcare providers are encouraged to wear gowns and gloves when examining patients with CF in order to limit the spread of virulent bacterial strains.<ref>Hoiby N. ''Isolation and treatment of cystic fibrosis patients with lung infections caused by Pseudomonas (Burkholderia) cepacia and multiresistant Pseudomonas aeruginosa.'' Neth J Med. 1995 Jun;46(6):280-7. PMID 7643943</ref> Often, patients with particularly damaging bacteria will attend clinics on different days and in different buildings than those without these infections. | ||
==See also== | ==See also== | ||
Revision as of 17:42, 3 February 2012
For patient information click here
| Cystic fibrosis | |
 | |
|---|---|
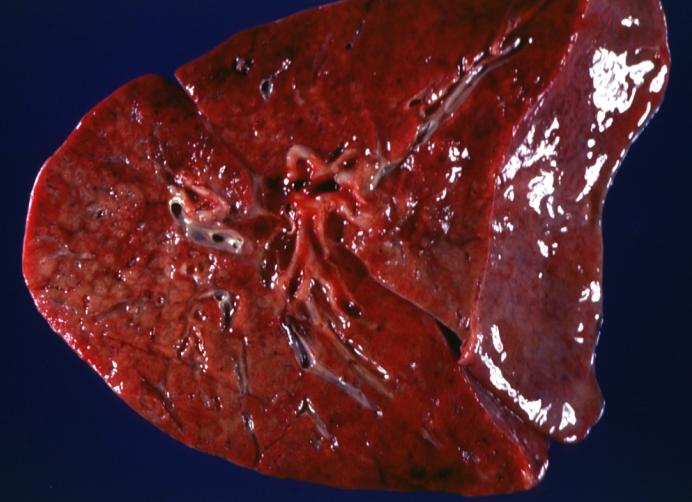
| Lung: Gross; Cystic fibrosis Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology | |
| ICD-10 | E84 |
| ICD-9 | 277 |
| OMIM | 219700 |
| DiseasesDB | 3347 |
| MedlinePlus | 000107 |
| MeSH | D003550 |
|
Cystic fibrosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Cystic fibrosis On the Web |
|
American Roentgen Ray Society Images of Cystic fibrosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Overview
Historical Perspective
Pathophysiology
Epidemiology & Demographics
Risk Factors
Screening
Causes
Differentiating Cystic fibrosis
Complications & Prognosis
Diagnosis
History and Symptoms | Physical Examination | Staging | Laboratory tests | Electrocardiogram | X Rays | CT | MRI Echocardiography or Ultrasound | Other images | Alternative diagnostics
Treatment
Medical therapy | Surgical options | Primary prevention | Secondary prevention | Financial costs | Future therapies
Diagnosis and monitoring
Cystic fibrosis may be diagnosed by many different categories of testing including those such as, newborn screening, sweat testing, or genetic testing. As of 2006 in the United States, 10 percent of cases are diagnosed shortly after birth as part of newborn screening programs. The newborn screen initially measures for raised blood concentration of immunoreactive trypsinogen.[1] However, most states and countries do not screen for CF routinely at birth. Therefore, most individuals are diagnosed after symptoms prompt an evaluation for cystic fibrosis. The most commonly-used form of testing is the sweat test. Sweat-testing involves application of a medication that stimulates sweating (pilocarpine) to one electrode of an apparatus and running electric current to a separate electrode on the skin. This process, called iontophoresis, causes sweating; the sweat is then collected on filter paper or in a capillary tube and analyzed for abnormal amounts of sodium and chloride. People with CF have increased amounts of sodium and chloride in their sweat. CF can also be diagnosed by identification of mutations in the CFTR gene.[2]
Prenatal diagnosis
Couples who are pregnant or who are planning a pregnancy can themselves be tested for CFTR gene mutations to determine the likelihood that their child will be born with cystic fibrosis. Testing is typically performed first on one or both parents and, if the risk of CF is found to be high, testing on the fetus can then be performed. Cystic fibrosis testing is offered to many couples in the US.[3] The American College of Obstetricians and Gynecologists (ACOG) recommends testing for couples who have a personal or close family history. Additionally, ACOG recommends that carrier testing be offered to all Caucasian couples and be made available to couples of other ethnic backgrounds.[4]
Because development of CF in the fetus requires each parent to pass on a mutated copy of the CFTR gene and because CF testing is expensive, testing is often performed on just one parent initially. If that parent is found to be a carrier of a CFTR gene mutation, the other parent is then tested to calculate the risk that their children will have CF. CF can result from more than a thousand different mutations and, as of 2006, it is not possible to test for each one. Testing analyzes the blood for the most common mutations such as ΔF508 — most commercially available tests look for 32 or fewer different mutations. If a family has a known uncommon mutation, specific screening for that mutation can be performed. Because not all known mutations are found on current tests, a negative screen does not guarantee that a child will not have CF.[5] In addition, because the mutations tested are necessarily those most common in the highest risk groups, testing in lower risk ethnicities is less successful because the mutations commonly seen in these groups are less common in the general population. These couples may therefore consider testing through labs that offer CF screens with a high number of mutations tested.
Couples who are at high risk for having a child with CF will often opt to perform further testing before or during pregnancy. In vitro fertilization with preimplantation genetic diagnosis offers the possibility to examine the embryo prior to its placement into the uterus. The test, performed 3 days after fertilization, looks for the presence of abnormal CF genes. If two mutated CFTR genes are identified, the embryo is not used for embryo transfer and an embryo with at least one normal gene is implanted.
During pregnancy, testing can be performed on the placenta (chorionic villus sampling) or the fluid around the fetus (amniocentesis). However, chorionic villus sampling has a risk of fetal death of 1 in 100 and amniocentesis of 1 in 200,[6] so the benefits must be determined to outweigh these risks prior to going forward with testing. Alternatively, some couples choose to undergo third party reproduction with egg or sperm donors.
The role of chronic infection in lung disease
The lungs of individuals with cystic fibrosis are colonized and infected by bacteria from an early age. These bacteria, which often spread amongst individuals with CF, thrive in the altered mucus, which collects in the small airways of the lungs. This mucus encourages the development of bacterial microenvironments (biofilms) that are difficult for immune cells (and antibiotics) to penetrate. The lungs respond to repeated damage by thick secretions and chronic infections by gradually remodeling the lower airways (bronchiectasis), making infection even more difficult to eradicate.[7]
Over time, both the types of bacteria and their individual characteristics change in individuals with CF. In the initial stage, common bacteria such as Staphylococcus aureus and Hemophilus influenzae colonize and infect the lungs. Eventually, however, Pseudomonas aeruginosa (and sometimes Burkholderia cepacia) dominates. Once within the lungs, these bacteria adapt to the environment and develop resistance to commonly used antibiotics. Pseudomonas can develop special characteristics that allow the formation of large colonies, known as "mucoid" Pseudomonas and rarely seen in people that do not have CF.[7]
One way in which infection has spread is by passage between different individuals with CF.[8] In the past, people with CF often participated in summer "CF Camps" and other recreational gatherings.[9][10] Hospitals grouped patients with CF into common areas and routine equipment (such as nebulizers)[11] was not sterilized between individual patients.[12] This led to transmission of more dangerous strains of bacteria among groups of patients. As a result, individuals with CF are routinely isolated from one another in the healthcare setting and healthcare providers are encouraged to wear gowns and gloves when examining patients with CF in order to limit the spread of virulent bacterial strains.[13] Often, patients with particularly damaging bacteria will attend clinics on different days and in different buildings than those without these infections.
See also
References
- ↑ Davies J et al. Cystic Fibrosis. BMJ. 2007 Dec 15;335(7632):1255–59.
- ↑ Stern, RC. The diagnosis of cystic fibrosis. N Engl J Med 1997; 336:487. PMID 9017943
- ↑ ACOG Committee Opinion #325: Update on Carrier Screening for Cystic Fibrosis. Obstet Gynecol 2005; 106:1465.
- ↑ American College of Obstetricians and Gynecologists and American College of Medical Genetics. Preconception and prenatal carrier screening for cystic fibrosis. Clinical and laboratory guidelines. American College of Obstetricians and Gynecologists, Washington, DC, October 2001.
- ↑ Elias, S, Annas, GJ, Simpson, JL. Carrier screening for cystic fibrosis: Implications for obstetric and gynecologic practice. Am J Obstet Gynecol 1991; 164:1077. PMID 2014829
- ↑ Tabor A, Philip J, Madsen M, Bang J, Obel EB, Norgaard-Pedersen B. Randomised controlled trial of genetic amniocentesis in 4606 low-risk women. Lancet. 1986 Jun 7;1(8493):1287–93. PMID 2423826
- ↑ 7.0 7.1 Saiman L. Microbiology of early CF lung disease. Paediatr Respir Rev. 2004;5 Suppl A:S367-9. PMID 14980298
- ↑ Tummler B, Koopmann U, Grothues D, Weissbrodt H, Steinkamp G, von der Hardt H. Nosocomial acquisition of Pseudomonas aeruginosa by cystic fibrosis patients. J Clin Microbiol. 1991 Jun;29(6):1265–7. PMID 1907611
- ↑ Centers for Disease Control and Prevention (CDC). Pseudomonas cepacia at summer camps for persons with cystic fibrosis. MMWR Morb Mortal Wkly Rep. 1993 Jun 18;42(23):456-9. PMID 7684813
- ↑ Pegues DA, Carson LA, Tablan OC, FitzSimmons SC, Roman SB, Miller JM, Jarvis WR.Acquisition of Pseudomonas cepacia at summer camps for patients with cystic fibrosis. Summer Camp Study Group. J Pediatr. 1994 May;124(5 Pt 1):694–702. PMID 7513755
- ↑ Pankhurst CL, Philpott-Howard J. The environmental risk factors associated with medical and dental equipment in the transmission of Burkholderia (Pseudomonas) cepacia in cystic fibrosis patients. J Hosp Infect. 1996 Apr;32(4):249-55. PMID 8744509
- ↑ Jones AM, Govan JR, Doherty CJ, Dodd ME, Isalska BJ, Stanbridge TN, Webb AK. Identification of airborne dissemination of epidemic multiresistant strains of Pseudomonas aeruginosa at a CF centre during a cross infection outbreak. Thorax. 2003 Jun;58(6):525-7. PMID 12775867
- ↑ Hoiby N. Isolation and treatment of cystic fibrosis patients with lung infections caused by Pseudomonas (Burkholderia) cepacia and multiresistant Pseudomonas aeruginosa. Neth J Med. 1995 Jun;46(6):280-7. PMID 7643943
External links
- Template:Dmoz
- Cystic Fibrosis Worldwide
- Cystic Fibrosis Foundation
- Cystic fibrosis pictures (Univ Geneva, Switzerland)
- cf at NIH/UW GeneTests
- Rare Diseases Clinical Research Network
- Search GeneCards for genes envolved in Cystic Fibrosis
Template:SIB
Template:Other metabolic pathology
Template:Link FA
ar:تليف كيسي ca:Fibrosi quística cs:Cystická fibróza cy:Ffibrosis systig da:Cystisk fibrose de:Mukoviszidose et:Tsüstiline fibroos eu:Fibrosi kistiko fa:فیبروز کیستیک it:Fibrosi cistica he:סיסטיק פיברוזיס hu:Cisztás fibrózis nl:Taaislijmziekte no:Cystisk fibrose nn:Cystisk fibrose sq:Fibroza kistike simple:Cystic fibrosis sk:Cystická fibróza sr:Цистична фиброза fi:Kystinen fibroosi sv:Cystisk fibros uk:Кістозний фіброз