Clorazepate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Turky Alkathery, M.D. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Clorazepate is a benzodiazepine that is FDA approved for the treatment of anxiety disorders and symptomatic relief of acute alcohol withdrawal. Common adverse reactions include drowsiness, dizziness, various gastrointestinal complaints, nervousness, blurred vision, dry mouth, headache, and mental confusion.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Symptomatic Relief of Anxiety
- Dosing information
- Clorazepate dipotassium tablets are administered orally in divided doses.
- Usual daily dose: 30 mg. The dose should be adjusted gradually within the range of 15 to 60 mg daily in accordance with the response of the patient. In elderly or debilitated patients it is advisable to initiate treatment at a daily dose of 7.5 to 15 mg.
- Clorazepate dipotassium tablets may also be administered in a single dose daily at bedtime
- Recommended initial dose: 15 mg. After the initial dose, the response of the patient may require adjustment of subsequent dosage. Lower doses may be indicated in the elderly patient. Drowsiness may occur at the initiation of treatment and with dosage increment.
Symptomatic Relief of Acute Alcohol Withdrawal
- Dosing information
- The following dosage schedule is recommended
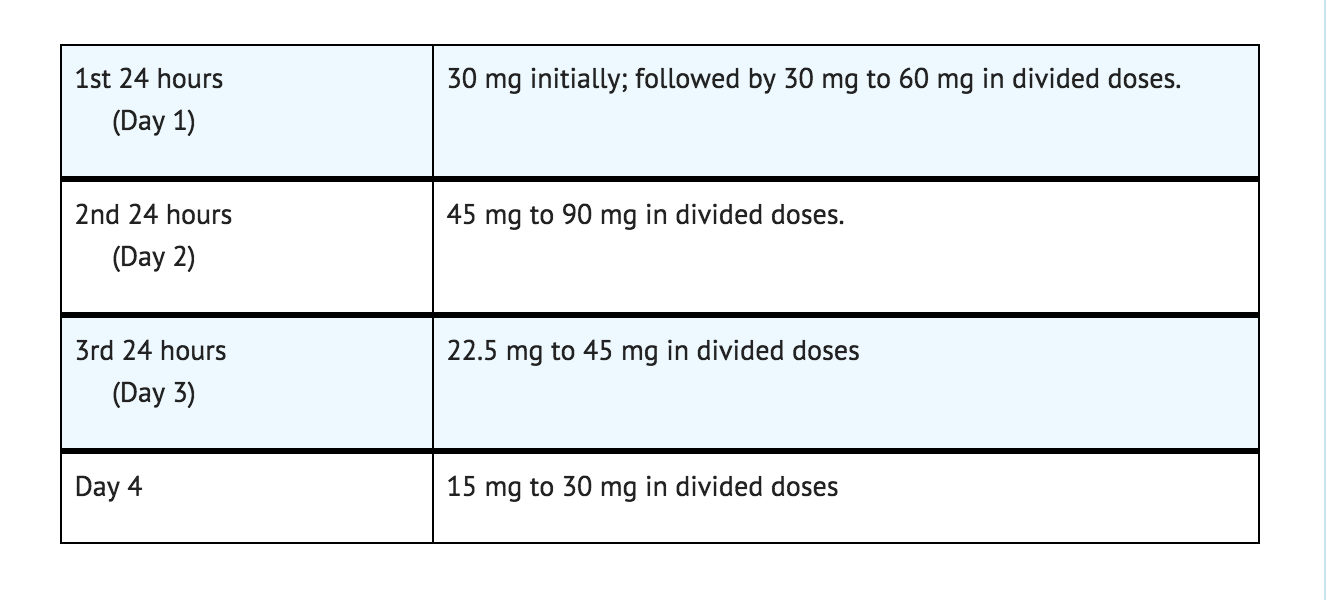
- Thereafter, gradually reduce the daily dose to 7.5 to 15 mg. Discontinue drug therapy as soon as patient's condition is stable.
- The maximum recommended total daily dose is 90 mg. Avoid excessive reductions in the total amount of drug administered on successive days.
Adjunct to Antiepileptic Drugs
- Dosing information
- In order to minimize drowsiness, the recommended initial dosages and dosage increments should not be exceeded.
- Maximum recommended initial dose in patients over 12 years old: 7.5 mg PO tid. Dosage should be increased by no more than 7.5 mg every week and should not exceed 90 mg/day.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of clorazepate in adult patients.
Non–Guideline-Supported Use
Epilepsy
- Dosing information
- 45 to 288 milligrams daily[1]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
For children (9-12 years)
- Dosing information
- Maximum recommended initial dose: 7.5 mg PO bid. Dosage should be increased by no more than 7.5 mg every week and should not exceed 60 mg/day.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of clorazepate in pediatric patients.
Non–Guideline-Supported Use
Epilepsy
- Dosing information
- 3.75 mg given 2 to 4 times daily[2]
Contraindications
- Clorazepate dipotassium tablets are contraindicated in patients with a known hypersensitivity to the drug and in those with acute narrow angle glaucoma.
Warnings
Use in Depressive Neuroses or Psychotic Reactions
- Clorazepate dipotassium tablets are not recommended for use in depressive neuroses or in psychotic reactions.
Interference with Psychomotor Performance
- Patients taking clorazepate dipotassium tablets should be cautioned against engaging in hazardous occupations requiring mental alertness, such as operating dangerous machinery including motor vehicles.
Concomitant Use with CNS Depressants
- Since clorazepate dipotassium has a central nervous system depressant effect, patients should be advised against the simultaneous use of other CNS depressant drugs, and cautioned that the effects of alcohol may be increased.
Physical and Psychological Dependence
- Withdrawal symptoms (similar in character to those noted with barbiturates and alcohol) have occurred following abrupt discontinuance of clorazepate. Withdrawal symptoms associated with the abrupt discontinuation of benzodiazepines have included convulsions, delirium, tremor, abdominal and muscle cramps, vomiting, sweating, nervousness, insomnia, irritability, diarrhea, and memory impairment. The more severe withdrawal symptoms have usually been limited to those patients who had received excessive doses over an extended period of time. Generally milder withdrawal symptoms have been reported following abrupt discontinuance of benzodiazepines taken continuously at therapeutic levels for several months. Consequently, after extended therapy, abrupt discontinuation of clorazepate should generally be avoided and a gradual dosage tapering schedule followed.
- Caution should be observed in patients who are considered to have a psychological potential for drug dependence.
- Evidence of drug dependence has been observed in dogs and rabbits which was characterized by convulsive seizures when the drug was abruptly withdrawn or the dose was reduced; the syndrome in dogs could be abolished by administration of clorazepate.
Suicidal Behavior and Ideation
- Antiepileptic drugs (AEDs), including clorazepate dipotassium, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
- The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
- The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed. Table 1 shows absolute and relative risk by indication for all evaluated AEDs.

- The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
- Anyone considering prescribing clorazepate dipotassium or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
- Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Adverse Reactions
Clinical Trials Experience
- The side effect most frequently reported was drowsiness. Less commonly reported (in descending order of occurrence) were: dizziness, various gastrointestinal complaints, nervousness, blurred vision, dry mouth, headache, and mental confusion. Other side effects included insomnia, transient skin rashes, fatigue, ataxia, genitourinary complaints, irritability, diplopia, depression, tremor and slurred speech.
- There have been reports of abnormal liver and kidney function tests and of decrease in hematocrit.
- Decrease in systolic blood pressure has been observed.
Postmarketing Experience
- There is limited information regarding Postmarketing Experience of Clorazepate Dipotassium
Drug Interactions
- If clorazepate dipotassium is to be combined with other drugs acting on the central nervous system, careful consideration should be given to the pharmacology of the agents to be employed. Animal experience indicates that clorazepate dipotassium prolongs the sleeping time after hexobarbital or after ethyl alcohol, increases the inhibitory effects of chlorpromazine, but does not exhibit monoamine oxidase inhibition. Clinical studies have shown increased sedation with concurrent hypnotic medications. The actions of the benzodiazepines may be potentiated by barbiturates, narcotics, phenothiazines, monoamine oxidase inhibitors or other antidepressants.
- If clorazepate dipotassium tablets are used to treat anxiety associated with somatic disease states, careful attention must be paid to possible drug interaction with concomitant medication.
- In bioavailability studies with normal subjects, the concurrent administration of antacids at therapeutic levels did not significantly influence the bioavailability of clorazepate dipotassium tablets.
Use in Specific Populations
Pregnancy
- An increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam, and meprobamate) during the first trimester of pregnancy has been suggested in several studies. Clorazepate dipotassium, a benzodiazepine derivative, has not been studied adequately to determine whether it, too, may be associated with an increased risk of fetal abnormality. Because use of these drugs is rarely a matter of urgency, their use during this period should almost always be avoided. The possibility that a woman of childbearing potential may be pregnant at the time of institution of therapy should be considered. Patients should be advised that if they become pregnant during therapy or intend to become pregnant they should communicate with their physician about the desirability of discontinuing the drug.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clorazepate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clorazepate during labor and delivery.
Nursing Mothers
- Clorazepate dipotassium tablets should not be given to nursing mothers since it has been reported that nordiazepam is excreted in human breast milk.
Pediatric Use
- Because of the lack of sufficient clinical experience, clorazepate dipotassium tablets are not recommended for use in patients less than 9 years of age.
Geriatic Use
- Clinical studies of clorazepate dipotassium studies were not adequate to determine whether subjects aged 65 and over respond differently than younger subjects. Elderly or debilitated patients may be especially sensitive to the effects of all benzodiazepines, including clorazepate dipotassium. In general, elderly or debilitated patients should be started on lower doses of clorazepate dipotassium tablets and observed closely, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy. Dose adjustments should also be made slowly, and with more caution in this patient population
Gender
There is no FDA guidance on the use of Clorazepate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clorazepate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clorazepate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Clorazepate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clorazepate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clorazepate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Patients treated with any anti-epileptic drug for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
- Monitoring of the vital signs and close observation for patient with clorazepate dipotassium overdose.
IV Compatibility
- There is limited information about the IV compatibility.
Overdosage
- Overdosage is usually manifested by varying degrees of CNS depression ranging from slight sedation to coma. As in the management of overdosage with any drug, it should be borne in mind that multiple agents may have been taken.
- The treatment of overdosage should consist of the general measures employed in the management of overdosage of any CNS depressant. Gastric evacuation either by the induction of emesis, lavage, or both, should be performed immediately. General supportive care, including frequent monitoring of the vital signs and close observation of the patient is indicated. Hypotension though rarely reported, may occur with large overdoses. In such cases the use of agents such as Levophed® Bitartrate (norepinephrine bitartrate injection, USP) or Aramine® Injection metaraminol bitartrate injection, USP) should be considered.
- While reports indicate that individuals have survived overdoses of clorazepate dipotassium as high as 450 to 675 mg, these doses are not necessarily an accurate indication of the amount of drug absorbed since the time interval between ingestion and the institution of treatment was not always known. Sedation in varying degrees was the most common physiological manifestation of clorazepate dipotassium overdosage. Deep coma when it occurred was usually associated with the ingestion of other drugs in addition to clorazepate dipotassium.
- Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be used in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose.
Pharmacology
| |
| |
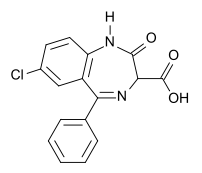
Clorazepate
| |
| Systematic (IUPAC) name | |
| 7-chloro-2,3-dihydro-2-oxo-5-phenyl- 1H-1,4-benzodiazepine-3-carboxylic acid | |
| Identifiers | |
| CAS number | 57109-90-7 (potassium salt) |
| ATC code | N05 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 314.72 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 91% |
| Metabolism | Hepatic |
| Half life | 48 hours |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
D(US) |
| Legal status |
Schedule IV(US) |
| Routes | Oral |
Mechanism of Action
- Pharmacologically, clorazepate dipotassium has the characteristics of the benzodiazepines. It has depressant effects on the central nervous system.
Structure
- Chemically, clorazepate dipotassium is a benzodiazepine. The empirical formula is C16H11ClK2N2O4; the molecular weight is 408.92; 1H-1, 4-Benzodiazepine-3-carboxylic acid, 7-chloro-2,3-dihydro-2-oxo-5-phenyl-, potassium salt compound with potassium hydroxide (1:1) and the structural formula may be represented as follows:

- The compound occurs as a fine, light yellow, practically odorless powder. It is insoluble in the common organic solvents, but very soluble in water. Aqueous solutions are unstable, clear, light yellow, and alkaline
Pharmacodynamics
- The primary metabolite, nordiazepam, quickly appears in the blood stream. The serum half-life is about 2 days. The drug is metabolized in the liver and excreted primarily in the urine. Studies in healthy men have shown that clorazepate dipotassium has depressant effects on the central nervous system. Prolonged administration of single daily doses as high as 120 mg was without toxic effects. Abrupt cessation of high doses was followed in some patients by nervousness, insomnia, irritability, diarrhea, muscle aches, or memory impairment.
Pharmacokinetics
- Since orally administered clorazepate dipotassium is rapidly decarboxylated to form nordiazepam, there is essentially no circulating parent drug. nordiazepam, the primary metabolite, quickly appears in the blood and is eliminated from the plasma with an apparent half-life of about 40 to 50 hours. Plasma levels of nordiazepam increase proportionally with Clorazepate Dipotassium dose and show moderate accumulation with repeated administration. The protein binding of nor diazepam in plasma is high (97-98%).
- Within 10 days after oral administration of a 15 mg (50µCi) dose of 14C-Clorazepate dipotassium to two volunteers, 62-67% of the radioactivity was excreted in the urine and 15-19% was eliminated in the feces. Both subjects were still excreting measurable amounts of radioactivity in the urine (about l% of the 14C-dose) on day ten.
- Nordiazepam is further metabolized by hydroxylation. The major urinary metabolite is conjugated oxazepam (3-hydroxynordiazepam), and smaller amounts of conjugated p-hydroxynordiazepam and nordiazepam are also found in the urine.
Nonclinical Toxicology
- Studies in rats and monkeys have shown a substantial difference between doses producing tranquilizing, sedative and toxic effects. In rats, conditioned avoidance response was inhibited at an oral dose of 10 mg/kg; sedation was induced at 32 mg/kg; the LD50 was 1320 mg/kg. In monkeys aggressive behavior was reduced at an oral dose of 0.25 mg/kg; sedation (ataxia) was induced at 7.5 mg/kg; the LD50 could not be determined because of the emetic effect of large doses, but the LD50 exceeds 1600 mg/kg.
- Twenty-four dogs were given clorazepate dipotassium orally in a 22-month toxicity study; doses up to 75 mg/kg were given. Drug-related changes occurred in the liver; weight was increased and cholestasis with minimal hepatocellular damage was found, but lobular architecture remained well preserved.
- Eighteen rhesus monkeys were given oral doses of clorazepate dipotassium from 3 to 36 mg/kg daily for 52 weeks. All treated animals remained similar to control animals. Although total leucocyte count remained within normal limits it tended to fall in the female animals on the highest doses.
- Examination of all organs revealed no alterations attributable to clorazepate dipotassium. There was no damage to liver function or structure.
Reproduction Studies
- Standard fertility, reproduction, and teratology studies were conducted in rats and rabbits. Oral doses in rats up to 150 mg/kg and in rabbits up to 15 mg/kg produced no abnormalities in the fetuses. Clorazepate dipotassium did not alter the fertility indices or reproductive capacity of adult animals. As expected, the sedative effect of high doses interfered with care of the young by their mothers
Clinical Studies
- FDA package insert for clorazepate contains no information regarding Clinical Studies.
How Supplied
- The 7.5 mg tablets are peach round, scored tablets debossed with M above the score and 40 below the score on one side of the tablet and blank on the other side. They are available as follows:
- NDC 67046-906-30
Storage
- Protect from moisture. Keep bottle tightly closed. Store between 20° to 25°C (68° to 70°F). Dispense in a USP tight, light-resistant container.
Images
Drug Images
{{#ask: Page Name::Clorazepate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Clorazepate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Clorazepate Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Clorazepate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- CLORAZEPATE DIPOTASSIUM ®[3]
- Gen-Xene
- Tranxene T-Tab
- Tranxene-SD
- Tranxene
Look-Alike Drug Names
There is limited information about the look alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Feldman RG (1976). "Chlorazepate in temporal lobe epilepsy". JAMA. 236 (23): 2603. PMID 11357.
- ↑ Naidu S, Gruener G, Brazis P (1986). "Excellent results with clorazepate in recalcitrant childhood epilepsies". Pediatr Neurol. 2 (1): 18–22. PMID 2907857.
- ↑ "CLORAZEPATE DIPOTASSIUM- clorazepate dipotassium tablet".
{{#subobject:
|Page Name=Clorazepate |Pill Name=Clorazepate_7.5 mg_NDC 0378-0040.jpg |Drug Name=Clorazepate Dipotassium 7.5 MG Oral Tablet |Pill Ingred=croscarmellose sodium, fd&c yellow no. 6, magnesium oxide, magnesium stearate, cellulose, microcrystalline, potassium carbonate, sodium chloride, sodium lauryl sulfate|+sep=; |Pill Imprint=M;40 |Pill Dosage=7.5 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=8.00 |Pill Scoring=2 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=0378-0040
}}
{{#subobject:
|Label Page=Clorazepate |Label Name=Clorazepate_label_01.jpg
}}
{{#subobject:
|Label Page=Clorazepate |Label Name=Clorazepate_panel_01.png
}}