Clarithromycin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: {[VP
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Clarithromycin is {{{aOrAn}}} {{{drugClass}}} that is FDA approved for the {{{indicationType}}} of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Clarithromycin FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Clarithromycin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
There is limited information regarding Clarithromycin Contraindications in the drug label.
Warnings
There is limited information regarding Clarithromycin Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clarithromycin Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Clarithromycin Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Clarithromycin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Clarithromycin in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clarithromycin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Clarithromycin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Clarithromycin in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Clarithromycin in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Clarithromycin in geriatric settings.
Gender
There is no FDA guidance on the use of Clarithromycin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clarithromycin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Clarithromycin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Clarithromycin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clarithromycin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clarithromycin in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Clarithromycin Administration in the drug label.
Monitoring
There is limited information regarding Clarithromycin Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Clarithromycin and IV administrations.
Overdosage
There is limited information regarding Clarithromycin overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Clarithromycin Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Clarithromycin Mechanism of Action in the drug label.
Structure
There is limited information regarding Clarithromycin Structure in the drug label.
Pharmacodynamics
There is limited information regarding Clarithromycin Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Clarithromycin Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Clarithromycin Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Clarithromycin Clinical Studies in the drug label.
How Supplied
There is limited information regarding Clarithromycin How Supplied in the drug label.
Storage
There is limited information regarding Clarithromycin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Clarithromycin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Clarithromycin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Clarithromycin Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Clarithromycin interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Clarithromycin Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Clarithromycin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
|genericName=
Clarithromycin
|aOrAn=
a
|drugClass=
macrolide antibiotic
|indication=
|hasBlackBoxWarning=
Yes
|adverseReactions=
|blackBoxWarningTitle=
Title
|blackBoxWarningBody= ConditionName:
- Content
|fdaLIADAdult=
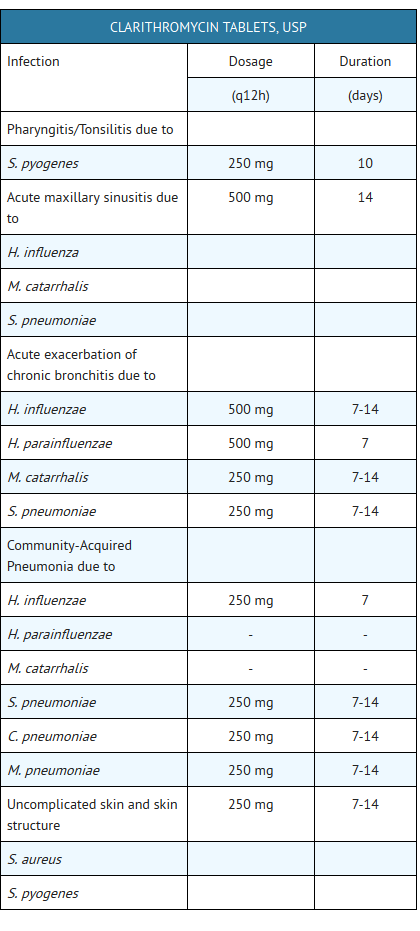
Pharyngitis/Tonsillitis
Pharyngitis/Tonsillitis due to Streptococcus pyogenes (The usual drug of choice in the treatment and prevention of streptococcal infections and the prophylaxis of rheumatic fever is penicillin administered by either the intramuscular or the oral route. Clarithromycin is generally effective in the eradication of S. pyogenes from the nasopharynx; however, data establishing the efficacy of clarithromycin in the subsequent prevention of rheumatic fever are not available at present.)
Acute maxillary sinusitis
Acute maxillary sinusitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcuspneumoniae
Acute bacterial exacerbation of chronic bronchitis
Acute bacterial exacerbation of chronic bronchitis due to Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis, or Streptococcus pneumoniae
Community-Acquired Pneumonia
Community-Acquired Pneumonia due to Haemophilus influenzae, Mycoplasma pneumoniae, Streptococcus pneumoniae, or Chlamydia pneumoniae (TWAR)
Uncomplicated Skin and Skin Structure Infections
Uncomplicated skin and skin structure infections due to Staphylococcus aureus, or Streptococcuspyogenes (Abscesses usually require surgical drainage.)
Disseminated Mycobacterial Infections=
Disseminated mycobacterial infections due to Mycobacterium avium, or Mycobacterium intracellulare
Active Duodenal Ulcer associated with H. pylori infection
- Clarithromycin tablets in combination with amoxicillin and lansoprazole or omeprazole delayed-release capsules, as triple therapy, are indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or five-year history of duodenal ulcer) to eradicate H. pylori.
- Clarithromycin tablets in combination with omeprazole capsules or ranitidine bismuth citrate tablets are also indicated for the treatment of patients with an active duodenal ulcer associated with H. pylori infection. However, regimens which contain clarithromycin as the single antimicrobial agent are more likely to be associated with the development of clarithromycin resistance among patients who fail therapy. Clarithromycin-containing regimens should not be used in patients with known or suspected clarithromycin resistant isolates because the efficacy of treatment is reduced in this setting.
- In patients who fail therapy, susceptibility testing should be done if possible. If resistance to clarithromycin is demonstrated, a non-clarithromycin-containing therapy is recommended. (For information on development of resistance see Microbiology section.) The eradication of H. pylori has been demonstrated to reduce the risk of duodenal ulcer recurrence.
Dosage and Administration
1
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
- Triple therapy: Clarithromycin/lansoprazole/amoxicillin
- The recommended adult dose is 500 mg clarithromycin, 30 mg lansoprazole, and 1 gram amoxicillin, all given twice daily (ql2h) for 10 or 14 days. (See INDICATIONS ANDUSAGE and CLINICAL STUDIES sections.)
- Triple therapy: Clarithromycin/omeprazole/amoxicillin
- The recommended adult dose is 500 mg clarithromycin, 20 mg omeprazole, and 1 gram amoxicillin, all given twice daily (ql2h) for 10 days. (See INDICATIONS AND USAGE and CLINICAL STUDIES sections.) In patients with an ulcer present at the time of initiation of therapy, an additional 18 days of omeprazole 20 mg once daily is recommended for ulcer healing and symptom relief.
- Dual therapy: Clarithromycin/ omeprazole
- The recommended adult dose is 500 mg clarithromycin given three times daily (q8h) and 40 mg omeprazole given once daily (qAM) for 14 days. (See INDICATIONS ANDUSAGE and CLINICAL STUDIES sections.) An additional 14 days of omeprazole 20 mg once daily is recommended for ulcer healing and symptom relief.
- Dual therapy: Clarithromycin/ranitidine bismuth citrate
- The recommended adult dose is 500 mg clarithromycin given twice daily (ql2h) or three times daily (q8h) and 400 mg ranitidine bismuth citrate given twice daily (ql2h) for 14 days. An additional 14 days of 400 mg twice daily is recommended for ulcer healing and symptom relief. Clarithromycin and ranitidine bismuth citrate combination therapy is not recommended in patients with creatinine clearance less than 25 mL/min. (See INDICATIONS AND USAGE and CLINICAL STUDIES sections.)
|offLabelAdultGuideSupport=
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Clarithromycin in adult patients.
|offLabelAdultNoGuideSupport=
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clarithromycin in adult patients.
|fdaLIADPed=
Pharyngitis/Tonsillitis
Pharyngitis/Tonsillitis due to Streptococcus pyogenes.
Acute maxillary sinusitis
Acute maxillary sinusitis due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcus pneumoniae
Acute otitis media
Acute otitis media due to Haemophilus influenzae, Moraxella catarrhalis, or Streptococcuspneumoniae
Community-Acquired Pneumonia
Community-Acquired Pneumonia due to Mycoplasma pneumoniae, Streptococcus pneumoniae, or Chlamydia pneumoniae (TWAR)
Uncomplicated Skin and Skin Structure Infections
Uncomplicated skin and skin structure infections due to Staphylococcus aureus, or Streptococcuspyogenes (Abscesses usually require surgical drainage.)
Disseminated Mycobacterial Infections=
- Disseminated mycobacterial infections due to Mycobacterium avium, or Mycobacteriumintracellulare
- Prophylaxis: Clarithromycin tablets are indicated for the prevention of disseminated Mycobacterium avium complex (MAC) disease in patients with advanced HIV infection.
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of clarithromycin and other antibacterial drugs, clarithromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Dosage and Administration
- The usual recommended daily dosage is 15 mg/kg/day divided ql2h for 10 days.
1
|offLabelPedGuideSupport=
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Clarithromycin in pediatric patients.
|offLabelPedNoGuideSupport=
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Clarithromycin in pediatric patients.
|contraindications=
- Clarithromycin is contraindicated in patients with a known hypersensitivity to clarithromycin, erythromycin, or any of the macrolide antibiotics.
- Concomitant administration of clarithromycin and any of the following drugs is contraindicated: cisapride, pimozide, astemizole, terfenadine, and ergotamine or dihydroergotamine (see Drug Interactions). There have been postmarketing reports of drug interactions when clarithromycin and/or erythromycin are coadministered with cisapride, pimozide, or terfenadine resulting in cardiac arrhythmias (QT prolongation, ventricular tachycardia, ventricular fibrillation, and torsades de pointes) most likely due to inhibition of metabolism of these drugs by erythromycin and clarithromycin. Fatalities have been reported. For information about contraindications of other drugs indicated in combination with clarithromycin, refer to the CONTRAINDICATIONS section of their package inserts.
|warnings=
- CLARITHROMYCIN SHOULD NOT BE USED IN PREGNANT WOMEN EXCEPT IN CLINICALCIRCUMSTANCES WHERE NO ALTERNATIVE THERAPY IS APPROPRIATE. IF PREGNANCYOCCURS WHILE TAKING THIS DRUG, THE PATIENT SHOULD BE APPRISED OF THEPOTENTIAL HAZARD TO THE FETUS. CLARITHROMYCIN HAS DEMONSTRATED ADVERSEEFFECTS OF PREGNANCY OUTCOME AND/OR EMBRYO-FETAL DEVELOPMENT INMONKEYS, RATS, MICE, AND RABBITS AT DOSES THAT PRODUCED PLASMA LEVELS 2TO 17 TIMES THE SERUM LEVELS ACHIEVED IN HUMANS TREATED AT THE MAXIMUMRECOMMENDED HUMAN DOSES. (See PRECAUTIONS - Pregnancy.)
- Pseudomembranous colitis has been reported with nearly all antibacterial agents,including clarithromycin, and may range in severity from mild to life threatening.Therefore, it is important to consider this diagnosis in patients who present withdiarrhea subsequent to the administration of antibacterial agents.
- Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is a primary cause of “antibiotic-associated colitis”.
- After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to discontinuation of the drug alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against Clostridium difficile colitis.
- There have been post-marketing reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, especially in the elderly, some of which occurred in patients with renal insufficiency. Deaths have been reported in some such patients. (See PRECAUTIONS).
Precautions
- Description
|clinicalTrials=
There is limited information regarding Clinical Trial Experience of Clarithromycin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
|postmarketing=
There is limited information regarding Postmarketing Experience of Clarithromycin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
|drugInteractions=
- Drug
- Description
|useInPregnancyFDA=
- Pregnancy Category
|useInPregnancyAUS=
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clarithromycin in women who are pregnant.
|useInLaborDelivery= There is no FDA guidance on use of Clarithromycin during labor and delivery.
|useInNursing= There is no FDA guidance on the use of Clarithromycin with respect to nursing mothers.
|useInPed= There is no FDA guidance on the use of Clarithromycin with respect to pediatric patients.
|useInGeri= There is no FDA guidance on the use of Clarithromycin with respect to geriatric patients.
|useInGender= There is no FDA guidance on the use of Clarithromycin with respect to specific gender populations.
|useInRace= There is no FDA guidance on the use of Clarithromycin with respect to specific racial populations.
|useInRenalImpair= There is no FDA guidance on the use of Clarithromycin in patients with renal impairment.
|useInHepaticImpair= There is no FDA guidance on the use of Clarithromycin in patients with hepatic impairment.
|useInReproPotential= There is no FDA guidance on the use of Clarithromycin in women of reproductive potentials and males.
|useInImmunocomp= There is no FDA guidance one the use of Clarithromycin in patients who are immunocompromised.
|administration=
- Oral
- Intravenous
|monitoring=
There is limited information regarding Monitoring of Clarithromycin in the drug label.
- Description
|IVCompat=
There is limited information regarding IV Compatibility of Clarithromycin in the drug label.
|overdose=
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Clarithromycin in the drug label.
|drugBox=
|mechAction=
|structure=
- Clarithromycin is a semi-synthetic macrolide antibiotic. Chemically, it is 6-O-methylerythromycin. The molecular formula is C38H69NO13, and the molecular weight is 747.96. The structural formula is:
- Clarithromycin is a white to off-white crystalline powder. It is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water.
- Clarithromycin is available as immediate-release tablets.
- Each white to off-white, oval, biconvex, film-coated tablet debossed “C250” or “C500” on one side and “G” on the other side contains 250 mg or 500 mg of clarithromycin and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, pregelatinized starch, stearic acid, titanium dioxide, triacetin, and vanillin.
|PD=
There is limited information regarding Pharmacodynamics of Clarithromycin in the drug label.
|PK=
- Clarithromycin is rapidly absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability of 250 mg clarithromycin tablets was approximately 50%. For a single 500 mg dose of clarithromycin, food slightly delays the onset of clarithromycin absorption, increasing the peak time from approximately 2 to 2.5 hours. Food also increases the clarithromycin peak plasma concentration by about 24%, but does not affect the extent of clarithromycin bioavailability. Food does not affect the onset of formation of the antimicrobially active metabolite, 14-OH clarithromycin or its peak plasma concentration but does slightly decrease the extent of metabolite formation, indicated by an 11% decrease in area under the plasma concentrationtime curve (AUC). Therefore, clarithromycin tablets may be given without regard to food.
- In nonfasting healthy human subjects (males and females), peak plasma concentrations were attained within 2 to 3 hours after oral dosing. Steady-state peak plasma clarithromycin concentrations were attained within 3 days and were approximately 1 to 2 mcg/mL with a 250 mg dose administered every 12 hours and 3 to 4 mcg/mL with a 500 mg dose administered every 8 to 12 hours. The elimination half-life of clarithromycin was about 3 to 4 hours with 250 mg administered every 12 hours but increased to 5 to 7 hours with 500 mg administered every 8 to 12 hours. The nonlinearity of clarithromycin pharmacokinetics is slight at the recommended doses of 250 mg and 500 mg administered every 8 to 12 hours. With a 250 mg every 12 hours dosing, the principal metabolite, 14-OH clarithromycin, attains a peak steady-state concentration of about 0.6 mcg/mL and has an elimination half-life of 5 to 6 hours. With a 500 mg every 8 to 12 hours dosing, the peak steady-state concentration of 14-OH clarithromycin is slightly higher (up to 1 mcg/mL), and its elimination half-life is about 7 to 9 hours. With any of these dosing regimens, the steady-state concentration of this metabolite is generally attained within 3 to 4 days.
- After a 250 mg tablet every 12 hours, approximately 20% of the dose is excreted in the urine as clarithromycin, while after a 500 mg tablet every 12 hours, the urinary excretion of clarithromycin is somewhat greater, approximately 30% . In comparison, after an oral dose of 250 mg (125 mg/5 mL) suspension every 12 hours, approximately 40% is excreted in urine as clarithromycin. The renal clearance of clarithromycin is, however, relatively independent of the dose size and approximates the normal glomerular filtration rate. The major metabolite found in urine is 14-OH clarithromycin, which accounts for an additional 10% to 15% of the dose with either a 250 mg or a 500 mg tablet administered every 12 hours.
- Steady-state concentrations of clarithromycin and 14-OH clarithromycin observed following administration of 500 mg doses of clarithromycin every 12 hours to adult patients with HIV infection were similar to those observed in healthy volunteers. In adult HIV-infected patients taking 500 - or 1000-mg doses of clarithromycin every 12 hours, steady-state clarithromycin Cmax values ranged from 2 to 4 mcg/mL and 5 to 10 mcg/mL respectively.
- The steady-state concentrations of clarithromycin in subjects with impaired hepatic function did not differ from those in normal subjects; however, the 14-OH clarithromycin concentrations were lower in the hepatically impaired subjects. The decreased formation of 14-OH clarithromycin was at least partially offset by an increase in renal clearance of clarithromycin in the subjects with impaired hepatic function when compared to healthy subjects.
- The pharmacokinetics of clarithromycin was also altered in subjects with impaired renal function. (See PRECAUTIONS and DOSAGE AND ADMINISTRATION.)
- Clarithromycin and the 14-OH clarithromycin metabolite distribute readily into body tissues and fluids. There are no data available on cerebrospinal fluid penetration. Because of high intracellular concentrations, tissue concentrations are higher than serum concentrations. Examples of tissue and serum concentrations are presented below.
- CONCENTRATION (after 250 mg q12h)
- Clarithromycin 500 mg every 8 hours was given in combination with omeprazole 40 mg daily to healthy adult males. The plasma levels of clarithromycin and 14-hydroxy-clarithromycin were increased by the concomitant administration of omeprazole. For clarithromycin, the mean Cmax was 10% greater, the mean Cmin was 27% greater, and the mean AUC0-8 was 15% greater when clarithromycin was administered with omeprazole than when clarithromycin was administered alone. Similar results were seen for 14-hydroxy-clarithromycin, the mean Cmax was 45% greater, the mean Cmin was 57% greater, and the mean AUC0-8 was 45% greater. Clarithromycin concentrations in the gastric tissue and mucus were also increased by concomitant administration of omeprazole.
Microbiology
- Clarithromycin exerts its antibacterial action by binding to the 50S ribosomal subunit of susceptible microorganisms resulting in inhibition of protein synthesis.
- Clarithromycin is active in vitro against a variety of aerobic and anaerobic gram-positive and gram-negative microorganisms as well as most Mycobacterium avium complex (MAC) microorganisms.
- Additionally, the 14-OH clarithromycin metabolite also has clinically significant antimicrobial activity. The 14-OH clarithromycin is twice as active against Haemophilus influenzae microorganisms as the parent compound. However, for Mycobacterium avium complex (MAC) isolates the 14-OH metabolite is 4 to 7 times less active than clarithromycin. The clinical significance of this activity against Mycobacterium avium complex is unknown.
- Clarithromycin has been shown to be active against most strains of the following microorganisms both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
- Aerobic Gram-positive microorganisms: Staphylococcus aureus, Streptococcus pneumoniae, Streptococcuspyogenes
- Aerobic Gram-negative microorganisms: Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis
- Other microorganisms: Mycoplasma pneumoniae, Chlamydia pneumoniae (TWAR) Mycobacteria: Mycobacterium avium complex (MAC) consisting of: Mycobacterium avium, Mycobacterium intracellulare
- Beta-lactamase production should have no effect on clarithromycin activity.
- NOTE: Most strains of methicillin-resistant and oxacillin-resistant staphylococci are resistant to clarithromycin.
- Omeprazole/clarithromycin dual therapy; ranitidine bismuth citrate/clarithromycin dual therapy; omeprazole/clarithromycin/amoxicillin triple therapy; and lansoprazole/clarithromycin/amoxicillin triple therapy have been shown to be active against most strains of Helicobacter pylori in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
- Helicobacter: Helicobacter pylori
Pretreatment Resistance
- Clarithromycin pretreatment resistance rates were 3.5% (4/113) in the omeprazole/clarithromycin dual therapy studies (M93-067, M93-l00) and 9.3% (41/439) in the omeprazole/clarithromycin/amoxicillin triple-therapy studies (126, 127, M96-446). Clarithromycin pretreatment resistance was 12.6% (44/348) in the ranitidine bismuth citrate/clarithromycin b.i.d. versus t.i.d. clinical study (H2BA3001). Clarithromycin pretreatment resistance rates were 9.5% (91/960) by E-test and 11.3% (12/106) by agar dilution in the lansoprazole/clarithromycin/amoxicillin triple therapy clinical trials (M93 -125, M93-130, M93-131, M95-392, and M95-399).
- Amoxicillin pretreatment susceptible isolates (<0.25 mcg/mL) were found in 99.3% (436/439) of the patients in the omeprazole/clarithromycin/amoxicillin clinical studies (126, 127, M96-446). Amoxicillin pretreatment minimum inhibitory concentrations (MICs) > 0.25 mcg/mL occurred in 0.7% (3/439) of the patients, all of whom were in the clarithromycin/amoxicillin study arm. Amoxicillin pretreatment susceptible isolates (< 0.25 mcg/mL) occurred in 97.8% (936/957) and 98.0% (98/100) of the patients in the lansoprazole/clarithromycin/amoxicillin triple-therapy clinical trials by E-test and agar dilution, respectively. Twenty-one of the 957 patients (2.2%) by Etest and 2 of 100 patients (2.0%) by agar dilution had amoxicillin pretreatment MICs of > 0.25 mcg/mL. Two patients had an unconfirmed pretreatment amoxicillin minimum inhibitory concentration (MIC) of > 256 mcg/mL by E-test.
- Clarithromycin Susceptibility Test Results and Clinical/Bacteriological Outcomes
- Patients not eradicated of H. pylori following omeprazole/clarithromycin, ranitidine bismuth citrate/clarithromycin, omeprazole/clarithromycin/amoxicillin, or lansoprazole/clarithromycin/amoxicillin therapy would likely have clarithromycin resistant H. pylori isolates. Therefore, for patients who fail therapy, clarithromycin susceptibility testing should be done, if possible. Patients with clarithromycin resistant H. pylori should not be treated with any of the following: omeprazole/clarithromycin dual therapy; ranitidine bismuth citrate/clarithromycin dual therapy; omeprazole/clarithromycin/amoxicillin triple therapy; lansoprazole/clarithromycin/amoxicillin triple therapy; or other regimens which include clarithromycin as the sole antimicrobial agent.
- Amoxicillin Susceptibility Test Results and Clinical/Bacteriological Outcomes
- In the omeprazole/clarithromycin/amoxicillin triple-therapy clinical trials, 84.9% (157/185) of the patients who had pretreatment amoxicillin susceptible MICs (< 0.25 mcg/mL) were eradicated of H. pylori and 15.1% (28/185) failed therapy. Of the 28 patients who failed triple therapy, 11 had no post-treatment susceptibility test results, and 17 had post-treatment H. pylori isolates with amoxicillin susceptible MICs. Eleven of the patients who failed triple therapy also had post-treatment H. pylori isolates with clarithromycin resistant MICs.
- In the lansoprazole/clarithromycin/amoxicillin triple-therapy clinical trials, 82.6% (195/236) of the patients that had pretreatment amoxicillin susceptible MICs (< 0.25 mcg/mL) were eradicated of H. pylori. Of those with pretreatment amoxicillin MICs of > 0.25 mcg/mL, three of six had the H. pylori eradicated. A total of 12.8% (22/172) of the patients failed the 10-and 14-day triple-therapy regimens. Post-treatment susceptibility results were not obtained on 11 of the patients who failed therapy. Nine of the 11 patients with amoxicillin post-treatment MICs that failed the triple-therapy regimen also had clarithromycin resistant H. pylori isolates.
- The following in vitro data are available, but their clinical significance is unknown. Clarithromycin exhibits in vitro activity against most strains of the following microorganisms; however, the safety and effectiveness of clarithromycin in treating clinical infections due to these microorganisms have not been established in adequate and well controlled clinical trials.
- Aerobic Gram-positive Microorganisms: Streptococcus agalactiae, Streptococci (Groups C, F, G), Viridans group streptococci
- Aerobic Gram-negative Microorganisms: Bordetella pertussis, Legionella pneumophila,Pasteurella multocida
- Anaerobic Gram-positive Microorganisms: Clostridium perfringens, Peptococcus niger,Propionibacterium acnes
- Anaerobic Gram-negative Microorganisms: Prevotella melaninogenica (formerly Bacteriodes melaninogenicus)
- Susceptibility Testing Excluding Mycobacteria and Helicobacter: Dilution Techniques
- Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized procedure. Standardized procedures are based on a dilution method1 (broth or agar) or equivalent with standardized inoculum concentrations and standardized concentrations of clarithromycin powder. The MIC values should be interpreted according to the following criteria:
- For testing Staphylococcus spp.
- For testing Streptococcus spp. including Streptococcus pneumoniae
- For testing Haemophilus spp.
- Note: When testing Streptococcus spp., including Streptococcus pneumoniae, susceptibility and resistance to clarithromycin can be predicted using erythromycin.
- A report of “Susceptible” indicates that the pathogen is likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable. A report of “Intermediate” indicates that the result should be considered equivocal, and, if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where high dosage of drug can be used. This category also provides a buffer zone which prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of “Resistant” indicates that the pathogen is not likely to be inhibited if the antimicrobial compound in the blood reaches the concentrations usually achievable; other therapy should be selected.
- Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard clarithromycin powder should provide the following MIC values:
1
- Diffusion Techniques
- Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. One such standardized procedure2 requires the use of standardized inoculum concentrations. This procedure uses paper disks impregnated with 15 mcg clarithromycin to test the susceptibility of microorganisms to clarithromycin.
- Reports from the laboratory providing results of the standard single-disk susceptibility test with a 15 mcg clarithromycin disk should be interpreted according to the following criteria:
- For testing Staphylococcus spp.
1
For testing Streptococcus spp. including Streptococcus pneumoniae
1
For testing Haemophilus spp.
1
- Note: When testing Streptococcus spp., including Streptococcus pneumoniae, susceptibility and resistance to clarithromycin can be predicted using erythromycin.
- Interpretation should be as stated above for results using dilution techniques. Interpretation involves correlation of the diameter obtained in the disk test with the MIC for clarithromycin.
- As with standardized dilution techniques, diffusion methods require the use of laboratory control microorganisms that are used to control the technical aspects of the laboratory procedures. For the diffusion technique, the 15 mcg clarithromycin disk should provide the following zone diameters in this laboratory test quality control strain:
1
- In Vitro Activity of Clarithromycin against Myobacteria
- Clarithromycin has demonstrated in vitro activity against Mycobacterium avium complex (MAC) microorganisms isolated from both AIDS and non-AIDS patients. While gene probe techniques may be used to distinguish M. avium species from M. intracellulare, many studies only reported results on M. avium complex (MAC) isolates
- Various in vitro methodologies employing broth or solid media at different pH’s, with and without oleic acid-albumin-dextrose-catalase (OADC), have been used to determine clarithromycin MIC values for mycobacterial species. In general, MIC values decrease more than 16-fold as the pH of Middlebrook 7H12 broth media increases from 5.0 to 7.4. At pH 7.4, MIC values determined with Mueller-Hinton agar were 4- to 8-fold higher than those observed with Middlebrook 7H12 media. Utilization of oleic acid-albumin-dextrose-catalase (OADC) in these assays has been shown to further alter MIC values.
- Clarithromycin activity against 80 MAC isolates from AIDS patients and 211 MAC isolates from non-AIDS patients was evaluated using a microdilution method with Middlebrook 7H9 broth. Results showed an MIC value of ≤ 4.0 mcg/mL in 81% and 89% of the AIDS and non- AIDS MAC isolates, respectively. Twelve percent of the non-AIDS isolates had an MIC value ≤ 0.5 mcg/mL. Clarithromycin was also shown to be active against phagocytized M. avium complex (MAC) in mouse and human macrophage cell cultures as well as in the beige mouse infection model.
- Clarithromycin activity was evaluated against Mycobacterium tuberculosis microorganisms. In one study utilizing the agar dilution method with Middlebrook 7H10 media, 3 of 30 clinical isolates had an MIC of 2.5 mcg/mL. Clarithromycin inhibited all isolates at > 10.0 mcg/
- Susceptibility Testing for Mycobacterium avium Complex (MAC): The disk diffusion and dilution techniques for susceptibility testing against gram-positive and gram-negative bacteria should not be used for determining clarithromycin MIC values against mycobacteria. In vitro susceptibility testing methods and diagnostic products currently available for determining minimum inhibitory concentration (MIC) values against Mycobacterium avium complex (MAC) organisms have not been standardized or validated. Clarithromycin MIC values will vary depending on the susceptibility testing method employed, composition and pH of the media, and the utilization of nutritional supplements. Breakpoints to determine whether clinical isolates of M. avium or M. intracellulare are susceptible or resistant to clarithromycin have not been established.
- Susceptibility Test for Helicobacter pylori: The reference methodology for susceptibility testing of H. pylori is agar dilution MICs3 One to three microliters of an inoculum equivalent to a No. 2 McFarland standard (1 x 107 - 1 x 108 CFU/mL for H. pylori) are inoculated directly onto freshly prepared antimicrobial containing Mueller-Hinton agar plates with 5% aged defibrinated sheep blood (> 2 weeks old). The agar dilution plates are incubated at 35°C in a microaerobic environment produced by a gas generating system suitable for Campylobacter species. After 3 days of incubation, the MICs are recorded as the lowest concentration of antimicrobial agent required to inhibit growth of the organism. The clarithromycin and amoxicillin MIC values should be interpreted according to the following criteria:
1
2
- Standardized susceptibility test procedures require the use of laboratory control microorganisms to control the technical aspects of the laboratory procedures. Standard clarithromycin and amoxicillin powders should provide the following MIC values:
1
|nonClinToxic=
There is limited information regarding Nonclinical Toxicology of Clarithromycin in the drug label.
|clinicalStudies=
There is limited information regarding Clinical Studies of Clarithromycin in the drug label.
|howSupplied=
|fdaPatientInfo=
There is limited information regarding Patient Counseling Information of Clarithromycin in the drug label.
|alcohol=
- Alcohol-Clarithromycin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
|brandNames=
- ®[1]
|lookAlike=
- A® — B®[2]
|drugShortage=
}}
{{#subobject:
|Page Name=Clarithromycin |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Clarithromycin |Label Name=Clarithromycin11.png
}}
{{#subobject:
|Label Page=Clarithromycin |Label Name=Clarithromycin11.png
}}
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)