Certolizumab pegol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS AND MALIGNANCY:
|
Overview
Certolizumab pegol is a tumor necrosis factor (TNF) blocker that is FDA approved for the {{{indicationType}}} of crohn's disease, rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. There is a Black Box Warning for this drug as shown here. Common adverse reactions include upper respiratory tract infection, rash, and urinary tract infection.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Crohn's Disease
- The recommended initial adult dose of CIMZIA is 400 mg (given as two subcutaneous injections of 200 mg) initially, and at Weeks 2 and 4. In patients who obtain a clinical response, the recommended maintenance regimen is 400 mg every four weeks.
Rheumatoid Arthritis
- The recommended dose of CIMZIA for adult patients with rheumatoid arthritis is 400 mg (given as two subcutaneous injections of 200 mg) initially and at Weeks 2 and 4, followed by 200 mg every other week. For maintenance dosing, CIMZIA 400 mg every 4 weeks can be considered [see Clinical Studies (14.2)].
Psoriatic Arthritis
- The recommended dose of CIMZIA for adult patients with psoriatic arthritis is 400 mg (given as 2 subcutaneous injections of 200 mg each) initially and at week 2 and 4, followed by 200 mg every other week. For maintenance dosing, CIMZIA 400 mg every 4 weeks can be considered [see Clinical Studies (14.3)].
Ankylosing Spondylitis
- The recommended dose of CIMZIA for adult patients with ankylosing spondylitis is 400 mg (given as 2 subcutaneous injections of 200 mg each) initially and at weeks 2 and 4, followed by 200 mg every 2 weeks or 400 mg every 4 weeks.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Certolizumab pegol in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Certolizumab pegol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Certolizumab pegol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Certolizumab pegol in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Certolizumab pegol in pediatric patients.
Contraindications
- None.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
SERIOUS INFECTIONS AND MALIGNANCY:
|
Precautions
- Risk of Serious Infections
- Patients treated with CIMZIA are at an increased risk for developing serious infections involving various organ systems and sites that may lead to hospitalization or death.
- Opportunistic infections due to bacterial, mycobacterial, invasive fungal, viral, parasitic, or other opportunistic pathogens including aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, histoplasmosis, legionellosis, listeriosis, pneumocystosis and tuberculosis have been reported with TNF blockers. Patients have frequently presented with disseminated rather than localized disease.
- Treatment with CIMZIA should not be initiated in patients with an active infection, including clinically important localized infections. Patients greater than 65 years of age, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants (e.g. corticosteroids or methotrexate) may be at a greater risk of infection. The risks and benefits of treatment should be considered prior to initiating therapy in patients:
- with chronic or recurrent infection
- who have been exposed to tuberculosis
- with a history of an opportunistic infection
- who have resided or traveled in areas of endemic tuberculosis or endemic mycoses, such as histoplasmosis, coccidioidomycosis, or blastomycosis
- with underlying conditions that may predispose them to infection
- Tuberculosis
- Cases of reactivation of tuberculosis or new tuberculosis infections have been observed in patients receiving CIMZIA, including patients who have previously received treatment for latent or active tuberculosis. Patients should be evaluated for tuberculosis risk factors and tested for latent infection prior to initiating CIMZIA and periodically during therapy.
- Treatment of latent tuberculosis infection prior to therapy with TNF-blocking agents has been shown to reduce the risk of tuberculosis reactivation during therapy. Induration of 5 mm or greater with tuberculin skin testing should be considered a positive test result when assessing if treatment for latent tuberculosis is needed prior to initiating CIMZIA, even for patients previously vaccinated with Bacille Calmette-Guerin (BCG).
- Anti-tuberculosis therapy should also be considered prior to initiation of CIMZIA in patients with a past history of latent or active tuberculosis in whom an adequate course of treatment cannot be confirmed, and for patients with a negative test for latent tuberculosis but having risk factors for tuberculosis infection. Consultation with a physician with expertise in the treatment of tuberculosis is recommended to aid in the decision of whether initiating anti-tuberculosis therapy is appropriate for an individual patient.
- Tuberculosis should be strongly considered in patients who develop a new infection during CIMZIA treatment, especially in patients who have previously or recently traveled to countries with a high prevalence of tuberculosis, or who have had close contact with a person with active tuberculosis.
- Monitoring
- Patients should be closely monitored for the development of signs and symptoms of infection during and after treatment with CIMZIA, including the development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy. Tests for latent tuberculosis infection may also be falsely negative while on therapy with CIMZIA.
- CIMZIA should be discontinued if a patient develops a serious infection or sepsis. A patient who develops a new infection during treatment with CIMZIA should be closely monitored, undergo a prompt and complete diagnostic workup appropriate for an immunocompromised patient, and appropriate antimicrobial therapy should be initiated.
- Invasive Fungal Infections
- For patients who reside or travel in regions where mycoses are endemic, invasive fungal infection should be suspected if they develop a serious systemic illness. Appropriate empiric antifungal therapy should be considered while a diagnostic workup is being performed. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. When feasible, the decision to administer empiric antifungal therapy in these patients should be made in consultation with a physician with expertise in the diagnosis and treatment of invasive fungal infections and should take into account both the risk for severe fungal infection and risks of antifungal therapy.
- Malignancies
- In the controlled portions of clinical studies of some TNF blockers, more cases of malignancies have been observed among patients receiving TNF blockers compared to control patients. During controlled and open-labeled portions of CIMZIA studies of Crohn's disease and other diseases, malignancies (excluding non-melanoma skin cancer) were observed at a rate (95% confidence interval) of 0.5 (0.4, 0.7) per 100 patient-years among 4,650 CIMZIA-treated patients versus a rate of 0.6 (0.1, 1.7) per 100 patient-years among 1,319 placebo-treated patients. The size of the control group and limited duration of the controlled portions of the studies precludes the ability to draw firm conclusions.
- Malignancies, some fatal, have been reported among children, adolescents, and young adults who received treatment with TNF-blocking agents (initiation of therapy ≤ 18 years of age), of which CIMZIA is a member. Approximately half the cases were lymphomas, including Hodgkin's and non-Hodgkin's lymphoma. The other cases represented a variety of different malignancies and included rare malignancies usually associated with immunosuppression and malignancies that are not usually observed in children and adolescents. The malignancies occurred after a median of 30 months of therapy (range 1 to 84 months). Most of the patients were receiving concomitant immunosuppressants. These cases were reported post-marketing and are derived from a variety of sources including registries and spontaneous post-marketing reports. CIMZIA is not indicated for use in pediatric patients.
- In the controlled portions of clinical trials of all the TNF blockers, more cases of lymphoma have been observed among patients receiving TNF blockers compared to control patients. In controlled studies of CIMZIA for Crohn's disease and other investigational uses, there was one case of lymphoma among 2,657 Cimzia-treated patients and one case of Hodgkin's lymphoma among 1,319 placebo-treated patients.
- In the CIMZIA RA clinical trials (placebo-controlled and open label) a total of three cases of lymphoma were observed among 2,367 patients. This is approximately 2-fold higher than expected in the general population. Patients with RA, particularly those with highly active disease, are at a higher risk for the development of lymphoma.
- Rates in clinical studies for CIMZIA cannot be compared to the rates of clinical trials of other TNF blockers and may not predict the rates observed when CIMZIA is used in a broader patient population. Patients with Crohn's disease that require chronic exposure to immunosuppressant therapies may be at higher risk than the general population for the development of lymphoma, even in the absence of TNF blocker therapy. The potential role of TNF blocker therapy in the development of malignancies in adults is not known.
- Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma that has a very aggressive disease course and is usually fatal, have been reported in patients treated with TNF blockers, including CIMZIA. The majority of reported TNF blocker cases occurred in adolescent and young adult males with Crohn's disease or ulcerative colitis. Almost all of these patients had received treatment with the immunosuppressants azathioprine and/or 6-mercaptopurine (6-MP) concomitantly with a TNF blocker at or prior to diagnosis. It is uncertain whether the occurrence of HSTCL is related to use of a TNF blocker or a TNF blocker in combination with these other immunosuppressants. The potential risk of using a TNF blocker in combination with azathioprine or 6-MP should be carefully considered.
- Cases of acute and chronic leukemia have been reported in association with post-marketing TNF-blocker use in RA and other indications. Even in the absence of TNF-blocker therapy, patients with RA may be at a higher risk (approximately 2-fold) than the general population for the development of leukemia.
- Periodic skin examinations are recommended for all patients, particularly those with risk factors for skin cancer.
- Heart Failure
- Cases of worsening congestive heart failure (CHF) and new onset CHF have been reported with TNF blockers, including CIMZIA. CIMZIA has not been formally studied in patients with CHF; however, in clinical studies in patients with CHF with another TNF blocker, worsening congestive heart failure (CHF) and increased mortality due to CHF were observed. Exercise caution in patients with heart failure and monitor them carefully.
- Hypersensitivity Reactions
- The following symptoms that could be compatible with hypersensitivity reactions have been reported rarely following CIMZIA administration to patients: angioedema, dyspnea, hypotension, rash, serum sickness, and urticaria. Some of these reactions occurred after the first administration of CIMZIA. If such reactions occur, discontinue further administration of CIMZIA and institute appropriate therapy. There are no data on the risks of using CIMZIA in patients who have experienced a severe hypersensitivity reaction towards another TNF blocker; in these patients caution is needed.
- Hepatitis B Virus Reactivation
- Use of TNF blockers, including CIMZIA, has been associated with reactivation of hepatitis B virus (HBV) in patients who are chronic carriers of this virus. In some instances, HBV reactivation occurring in conjunction with TNF blocker therapy has been fatal. The majority of reports have occurred in patients concomitantly receiving other medications that suppress the immune system, which may also contribute to HBV reactivation.
- Test patients for HBV infection before initiating treatment with CIMZIA. For patients who test positive for HBV infection, consultation with a physician with expertise in the treatment of hepatitis B is recommended. Adequate data are not available on the safety or efficacy of treating patients who are carriers of HBV with anti-viral therapy in conjunction with TNF blocker therapy to prevent HBV reactivation. Patients who are carriers of HBV and require treatment with CIMZIA should be closely monitored for clinical and laboratory signs of active HBV infection throughout therapy and for several months following termination of therapy.
- In patients who develop HBV reactivation, discontinue CIMZIA and initiate effective anti-viral therapy with appropriate supportive treatment. The safety of resuming TNF blocker therapy after HBV reactivation is controlled is not known. Therefore, exercise caution when considering resumption of CIMZIA therapy in this situation and monitor patients closely.
- Neurologic Reactions
- Use of TNF blockers, of which CIMZIA is a member, has been associated with rare cases of new onset or exacerbation of clinical symptoms and/or radiographic evidence of central nervous system demyelinating disease, including multiple sclerosis, and with peripheral demyelinating disease, including Guillain-Barré syndrome . Exercise caution in considering the use of CIMZIA in patients with pre-existing or recent-onset central or peripheral nervous system demyelinating disorders. Rare cases of neurological disorders, including seizure disorder, optic neuritis, and peripheral neuropathy have been reported in patients treated with CIMZIA.
- Hematological Reactions
- Rare reports of pancytopenia, including aplastic anemia, have been reported with TNF blockers. Adverse reactions of the hematologic system, including medically significant cytopenia (e.g., leukopenia, pancytopenia, thrombocytopenia) have been infrequently reported with CIMZIA. The causal relationship of these events to CIMZIA remains unclear.
- Although no high risk group has been identified, exercise caution in patients being treated with CIMZIA who have ongoing, or a history of, significant hematologic abnormalities. Advise all patients to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever, bruising, bleeding, pallor) while on CIMZIA. Consider discontinuation of CIMZIA therapy in patients with confirmed significant hematologic abnormalities.
- Use with Biological Disease-Modifying Antirheumatic Drugs (Biological DMARDs)
- Serious infections were seen in clinical studies with concurrent use of anakinra (an interleukin-1 antagonist) and another TNF blocker, etanercept, with no added benefit compared to etanercept alone. A higher risk of serious infections was also observed in combination use of TNF blockers with abatacept and rituximab. Because of the nature of the adverse events seen with this combination therapy, similar toxicities may also result from the use of CIMZIA in this combination. Therefore, the use of CIMZIA in combination with other biological DMARDs is not recommended.
- Autoimmunity
- Treatment with CIMZIA may result in the formation of autoantibodies and rarely, in the development of a lupus-like syndrome. If a patient develops symptoms suggestive of a lupus-like syndrome following treatment with CIMZIA, discontinue treatment.
- Immunizations
- Patients treated with CIMZIA may receive vaccinations, except for live or live attenuated vaccines. No data are available on the response to live vaccinations or the secondary transmission of infection by live vaccines in patients receiving CIMZIA.
- In a placebo-controlled clinical trial of patients with rheumatoid arthritis, no difference was detected in antibody response to vaccine between CIMZIA and placebo treatment groups when the pneumococcal polysaccharide vaccine and influenza vaccine were administered concurrently with CIMZIA. Similar proportions of patients developed protective levels of anti-vaccine antibodies between CIMZIA and placebo treatment groups; however patients receiving CIMZIA and concomitant methotrexate had a lower humoral response compared with patients receiving CIMZIA alone. The clinical significance of this is unknown.
- Immunosuppression
- Since TNF mediates inflammation and modulates cellular immune responses, the possibility exists for TNF blockers, including CIMZIA, to affect host defenses against infections and malignancies. The impact of treatment with CIMZIA on the development and course of malignancies, as well as active and/or chronic infections, is not fully understood. The safety and efficacy of CIMZIA in patients with immunosuppression has not been formally evaluated.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying and controlled conditions, adverse reaction rates observed in clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug, and may not predict the rates observed in a broader patient population in clinical practice.
- In premarketing controlled trials of all patient populations combined the most common adverse reactions (≥ 8%) were upper respiratory infections (18%), rash (9%) and urinary tract infections (8%).
Adverse Reactions Most Commonly Leading to Discontinuation of Treatment in Premarketing Controlled Trials
- The proportion of patients with Crohn's disease who discontinued treatment due to adverse reactions in the controlled clinical studies was 8% for CIMZIA and 7% for placebo. The most common adverse reactions leading to the discontinuation of CIMZIA (for at least 2 patients and with a higher incidence than placebo) were abdominal pain (0.4% CIMZIA, 0.2% placebo), diarrhea (0.4% CIMZIA, 0% placebo), and intestinal obstruction (0.4% CIMZIA, 0% placebo).
- The proportion of patients with rheumatoid arthritis who discontinued treatment due to adverse reactions in the controlled clinical studies was 5% for CIMZIA and 2.5% for placebo. The most common adverse reactions leading to discontinuation of CIMZIA were tuberculosis infections (0.5%); and pyrexia, urticaria, pneumonia, and rash (0.3%).
Controlled Studies with Crohn's Disease
- The data described below reflect exposure to CIMZIA at 400 mg subcutaneous dosing in studies of patients with Crohn's disease. In the safety population in controlled studies, a total of 620 patients with Crohn's disease received CIMZIA at a dose of 400 mg, and 614 subjects received placebo (including subjects randomized to placebo in Study CD2 following open label dosing of CIMZIA at Weeks 0, 2, 4). In controlled and uncontrolled studies, 1,564 patients received CIMZIA at some dose level, of whom 1,350 patients received 400 mg CIMZIA. Approximately 55% of subjects were female, 45% were male, and 94% were Caucasian. The majority of patients in the active group were between the ages of 18 and 64.
- During controlled clinical studies, the proportion of patients with serious adverse reactions was 10% for CIMZIA and 9% for placebo. The most common adverse reactions (occurring in ≥ 5% of CIMZIA-treated patients, and with a higher incidence compared to placebo) in controlled clinical studies with CIMZIA were upper respiratory infections (e.g. nasopharyngitis, laryngitis, viral infection) in 20% of CIMZIA-treated patients and 13% of placebo-treated patients, urinary tract infections (e.g. bladder infection, bacteriuria, cystitis) in 7% of CIMZIA-treated patients and in 6% of placebo-treated patients, and arthralgia (6% CIMZIA, 4% placebo).
Other Adverse Reactions
- The most commonly occurring adverse reactions in controlled trials of Crohn's disease were described above. Other serious or significant adverse reactions reported in controlled and uncontrolled studies in Crohn's disease and other diseases, occurring in patients receiving CIMZIA at doses of 400 mg or other doses include:
Blood and lymphatic system disorders
Anemia, leukopenia, lymphadenopathy, pancytopenia, and thrombophilia.
Cardiac disorders
Angina pectoris, arrhythmias, atrial fibrillation, cardiac failure, hypertensive heart disease, myocardial infarction, myocardial ischemia, pericardial effusion, pericarditis, stroke and transient ischemic attack.
Eye disorders
Optic neuritis, retinal hemorrhage, and uveitis.
General disorders and administration site conditions
Bleeding and injection site reactions.
Hepatobiliary disorders
Elevated liver enzymes and hepatitis.
Immune system disorders
Alopecia totalis.
Psychiatric disorders
Anxiety, bipolar disorder, and suicide attempt.
Renal and urinary disorders
Nephrotic syndrome and renal failure.
Reproductive system and breast disorders
Menstrual disorder.
Skin and subcutaneous tissue disorders
Dermatitis, erythema nodosum, and urticaria.
Vascular disorders
Thrombophlebitis, vasculitis.
Controlled Studies with Rheumatoid Arthritis
- CIMZIA was studied primarily in placebo-controlled trials and in long-term follow-up studies. The data described below reflect the exposure to CIMZIA in 2,367 RA patients, including 2,030 exposed for at least 6 months, 1,663 exposed for at least one year and 282 for at least 2 years; and 1,774 in adequate and well-controlled studies. In placebo-controlled studies, the population had a median age of 53 years at entry; approximately 80% were females, 93% were Caucasian and all patients were suffering from active rheumatoid arthritis, with a median disease duration of 6.2 years. Most patients received the recommended dose of CIMZIA or higher.
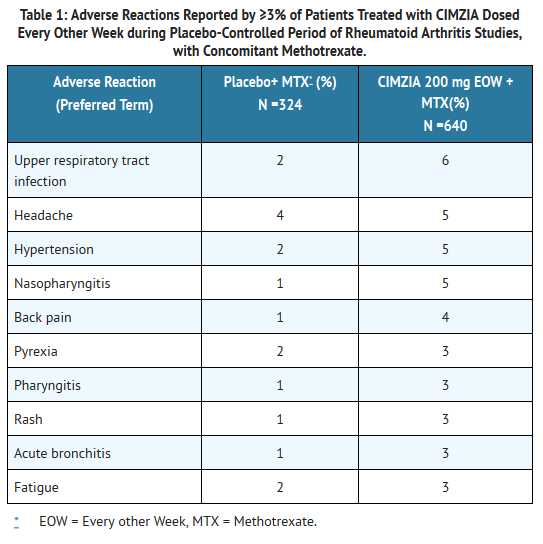
- Table 1 summarizes the reactions reported at a rate of at least 3% in patients treated with CIMZIA 200 mg every other week compared to placebo (saline formulation), given concomitantly with methotrexate.
T1
- Hypertensive adverse reactions were observed more frequently in patients receiving CIMZIA than in controls. These adverse reactions occurred more frequently among patients with a baseline history of hypertension and among patients receiving concomitant corticosteroids and non-steroidal anti-inflammatory drugs.
- Patients receiving CIMZIA 400 mg as monotherapy every 4 weeks in rheumatoid arthritis controlled clinical trials had similar adverse reactions to those patients receiving CIMZIA 200 mg every other week.
Other Adverse Reactions
- Other infrequent adverse reactions (occurring in less than 3% of RA patients) were similar to those seen in Crohn's disease patients.
Psoriatic Arthritis Clinical Study
- CIMZIA has been studied in 409 patients with psoriatic arthritis (PsA) in a placebo-controlled trial. The safety profile for patients with PsA treated with CIMZIA was similar to the safety profile seen in patients with RA and previous experience with CIMZIA.
Ankylosing Spondylitis Clinical Study
- CIMZIA has been studied in 325 patients with axial spondyloarthritis of whom the majority had ankylosing spondylitis (AS) in a placebo-controlled study (AS-1). The safety profile for patients in study AS-1 treated with CIMZIA was similar to the safety profile seen in patients with RA.
- Infections
- The incidence of infections in controlled studies in Crohn's disease was 38% for CIMZIA-treated patients and 30% for placebo-treated patients. The infections consisted primarily of upper respiratory infections (20% for CIMZIA, 13% for placebo). The incidence of serious infections during the controlled clinical studies was 3% per patient-year for CIMZIA-treated patients and 1% for placebo-treated patients. Serious infections observed included bacterial and viral infections, pneumonia, and pyelonephritis.
- The incidence of new cases of infections in controlled clinical studies in rheumatoid arthritis was 0.91 per patient-year for all CIMZIA-treated patients and 0.72 per patient-year for placebo-treated patients. The infections consisted primarily of upper respiratory tract infections, herpes infections, urinary tract infections, and lower respiratory tract infections. In the controlled rheumatoid arthritis studies, there were more new cases of serious infection adverse reactions in the CIMZIA treatment groups, compared to the placebo groups (0.06 per patient-year for all CIMZIA doses vs. 0.02 per patient-year for placebo). Rates of serious infections in the 200 mg every other week dose group were 0.06 per patient-year and in the 400 mg every 4 weeks dose group were 0.04 per patient-year. Serious infections included tuberculosis, pneumonia, cellulitis, and pyelonephritis. In the placebo group, no serious infection occurred in more than one subject. There is no evidence of increased risk of infections with continued exposure over time [see Warnings and Precautions (5.1)].
- Tuberculosis and Opportunistic Infections
- In completed and ongoing global clinical studies in all indications including 5,118 CIMZIA-treated patients, the overall rate of tuberculosis is approximately 0.61 per 100 patient-years across all indications.
- The majority of cases occurred in countries with high endemic rates of TB. Reports include cases of miliary, lymphatic, peritoneal, as well as pulmonary TB. The median time to onset of TB for all patients exposed to CIMZIA across all indications was 345 days. In the studies with CIMZIA in RA, there were 36 cases of TB among 2,367 exposed patients, including some fatal cases. Rare cases of opportunistic infections have also been reported in these clinical trials. [see Warnings and Precautions (5.1)].
- Malignancies
- In clinical studies of CIMZIA, the overall incidence rate of malignancies was similar for CIMZIA-treated and control patients. For some TNF blockers, more cases of malignancies have been observed among patients receiving those TNF blockers compared to control patients. [see Warnings and Precautions (5.2)]
- Heart Failure
- In placebo-controlled and open-label rheumatoid arthritis studies, cases of new or worsening heart failure have been reported for CIMZIA-treated patients. The majority of these cases were mild to moderate and occurred during the first year of exposure. [see Warnings and Precautions (5.3)].
- Autoantibodies
- In clinical studies in Crohn's disease, 4% of patients treated with CIMZIA and 2% of patients treated with placebo that had negative baseline ANA titers developed positive titers during the studies. One of the 1,564 Crohn's disease patients treated with CIMZIA developed symptoms of a lupus-like syndrome.
- In clinical trials of TNF blockers, including CIMZIA, in patients with RA, some patients have developed ANA. Four patients out of 2,367 patients treated with CIMZIA in RA clinical studies developed clinical signs suggestive of a lupus-like syndrome. The impact of long-term treatment with CIMZIA on the development of autoimmune diseases is unknown [see Warnings and Precautions (5.9)].
- Immunogenicity
- Patients were tested at multiple time points for antibodies to certolizumab pegol during Studies CD1 and CD2. The overall percentage of antibody positive patients was 8% in patients continuously exposed to CIMZIA, approximately 6% were neutralizing in vitro. No apparent correlation of antibody development to adverse events or efficacy was observed. Patients treated with concomitant immunosuppressants had a lower rate of antibody development than patients not taking immunosuppressants at baseline (3% and 11%, respectively). The following adverse events were reported in Crohn's disease patients who were antibody-positive (N = 100) at an incidence at least 3% higher compared to antibody-negative patients (N = 1,242): abdominal pain, arthralgia, edema peripheral, erythema nodosum, injection site erythema, injection site pain, pain in extremity, and upper respiratory tract infection.
- The overall percentage of patients with antibodies to certolizumab pegol detectable on at least one occasion was 7% (105 of 1,509) in the rheumatoid arthritis placebo-controlled trials. Approximately one third (3%, 39 of 1,509) of these patients had antibodies with neutralizing activity in vitro. Patients treated with concomitant immunosuppressants (MTX) had a lower rate of antibody development than patients not taking immunosuppressants at baseline. Patients treated with concomitant immunosuppressant therapy (MTX) in RA-I, RA-II, RA-III had a lower rate of neutralizing antibody formation overall than patients treated with CIMZIA monotherapy in RA-IV (2% vs. 8%). Both the loading dose of 400 mg every other week at Weeks 0, 2 and 4 and concomitant use of MTX were associated with reduced immunogenicity.
- Antibody formation was associated with lowered drug plasma concentration and reduced efficacy. In patients receiving the recommended CIMZIA dosage of 200 mg every other week with concomitant MTX, the ACR20 response was lower among antibody positive patients than among antibody-negative patients (Study RA-I, 48% versus 60%; Study RA-II 35% versus 59%, respectively). In Study RA-III, too few patients developed antibodies to allow for meaningful analysis of ACR20 response by antibody status. In Study RA-IV (monotherapy), the ACR20 response was 33% versus 56%, antibody-positive versus antibody-negative status, respectively. [see Clinical Pharmacology (12.3)]. No association was seen between antibody development and the development of adverse events.
- The data reflect the percentage of patients whose test results were considered positive for antibodies to certolizumab pegol in an ELISA, and are highly dependent on the sensitivity and specificity of the assay. The observed incidence of antibody (including neutralizing antibody) positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to certolizumab pegol with the incidence of antibodies to other products may be misleading.
- Hypersensitivity Reactions
- The following symptoms that could be compatible with hypersensitivity reactions have been reported rarely following CIMZIA administration to patients: angioedema, dermatitis allergic, dizziness (postural), dyspnea, hot flush, hypotension, injection site reactions, malaise, pyrexia, rash, serum sickness, and (vasovagal) syncope [see Warnings and Precautions (5.4)].
Postmarketing Experience
- The following adverse reactions have been identified during post-approval use of CIMZIA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to estimate reliably their frequency or establish a causal relationship to drug exposure.
Vascular disorder
Systemic vasculitis has been identified during post-approval use of TNF blockers.
Skin
Case of severe skin reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, erythema multiforme, and new or worsening psoriasis (all sub-types including pustular and palmoplantar) have been identified during post-approval use of TNF blockers.
Immune System Disorders
Sarcoidosis
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Certolizumab pegol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Certolizumab pegol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Certolizumab pegol with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Certolizumab pegol with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Certolizumab pegol with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Certolizumab pegol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Certolizumab pegol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Certolizumab pegol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Certolizumab pegol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Certolizumab pegol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Certolizumab pegol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Certolizumab pegol in the drug label.
- Description
IV Compatibility
- CIMZIA Lyophilized powder should be prepared and administered by a health care professional. CIMZIA is provided in a package that contains everything required to reconstitute and inject the drug. Step-by-step preparation and administration instructions are provided below.
- Preparation and Storage
- CIMZIA should be brought to room temperature before reconstituting.
- Use appropriate aseptic technique when preparing and administering CIMZIA.
- Reconstitute the vial(s) of CIMZIA with 1 mL of Sterile Water for Injection, USP using the 20-gauge needle provided.
- Gently swirl each vial of CIMZIA without shaking, assuring that all of the powder comes in contact with the Sterile Water for Injection.
- Leave the vial(s) undisturbed to fully reconstitute, which may take approximately 30 minutes.
- The final reconstituted solution contains 200 mg/mL and should be clear to opalescent, colorless to pale yellow liquid essentially free from particulates.
- Once reconstituted, CIMZIA can be stored in the vials for up to 24 hours between 2° to 8° C (36° to 46° F) prior to injection. Do not freeze.
- Administration
- Prior to injecting, reconstituted CIMZIA should be at room temperature but do not leave reconstituted CIMZIA at room temperature for more than two hours prior to administration.
- Withdraw the reconstituted solution into a separate syringe for each vial using a new 20-gauge needle for each vial so that each syringe contains 1 mL of CIMZIA (200 mg of certolizumab pegol).
- Replace the 20-gauge needle(s) on the syringes with a 23-gauge(s) for administration.
- Inject the full contents of the syringe(s) subcutaneously into thigh or abdomen. Where a 400 mg dose is required, two injections are required, therefore, separate sites should be used for each 200 mg injection.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Certolizumab pegol in the drug label.
Pharmacology
There is limited information regarding Certolizumab pegol Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Certolizumab pegol in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Certolizumab pegol in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Certolizumab pegol in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Certolizumab pegol in the drug label.
How Supplied
Storage
There is limited information regarding Certolizumab pegol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Certolizumab pegol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Certolizumab pegol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Certolizumab pegol in the drug label.
Precautions with Alcohol
- Alcohol-Certolizumab pegol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Certolizumab pegol |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Certolizumab pegol |Label Name=Certolizumab pegol11.png
}}
{{#subobject:
|Label Page=Certolizumab pegol |Label Name=Certolizumab pegol11.png
}}