Ceritinib: Difference between revisions
(Created page with "{{DrugProjectFormSinglePage |authorTag= <!--Overview--> |genericName= |aOrAn= a |drugClass= |indication= |hasBlackBoxWarning= Yes |adverseReactions= <!--Bla...") |
No edit summary |
||
| Line 45: | Line 45: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Non-small cell lung cancer ===== | ||
*ZYKADIA is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib. | |||
*This indication is approved under accelerated approval based on tumor response rate and duration of response [see Clinical Studies (14)]. An improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. | |||
=====Dosing and Administration===== | |||
*The recommended dose of ZYKADIA is 750 mg orally once daily until disease progression or unacceptable toxicity. Administer ZYKADIA on an empty stomach (i.e., do not administer within 2 hours of a meal) [see Clinical Pharmacology (12.3)]. | |||
* | *A recommended dose has not been determined for patients with moderate to severe hepatic impairment [see Use in Specific Populations (8.6)]. | ||
*If a dose of ZYKADIA is missed, make up that dose unless the next dose is due within 12 hours. | |||
===== | =====Dose Modifications for Adverse Reactions===== | ||
* | *Recommendations for dose modifications of ZYKADIA for adverse reactions are provided in Table 1. | ||
*Approximately 60% of patients initiating treatment at the recommended dose required at least one dose reduction and the median time to first dose reduction was 7 weeks. | |||
*Discontinue ZYKADIA for patients unable to tolerate 300 mg daily. | |||
TABLE02 | |||
=====Dose Modification for Strong CYP3A4 Inhibitors===== | |||
*Avoid concurrent use of strong CYP3A inhibitors during treatment with ZYKADIA [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. | |||
* | *If concomitant use of a strong CYP3A inhibitor is unavoidable, reduce the ZYKADIA dose by approximately one-third, rounded to the nearest 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the ZYKADIA dose that was taken prior to initiating the strong CYP3A4 inhibitor. | ||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 161: | Line 166: | ||
|contraindications= | |contraindications= | ||
* | * None | ||
<!--Warnings--> | <!--Warnings--> | ||
| Line 167: | Line 172: | ||
|warnings= | |warnings= | ||
=====Severe or Persistent Gastrointestinal Toxicity===== | |||
==== | |||
*Diarrhea, nausea, vomiting, or abdominal pain occurred in 96% of 255 patients including severe cases in 14% of patients treated with ZYKADIA in Study 1. Dose modification due to diarrhea, nausea, vomiting, or abdominal pain occurred in 38% of patients. | |||
*Monitor and manage patients using standards of care, including anti-diarrheals, anti-emetics, or fluid replacement, as indicated. Based on the severity of the adverse drug reaction, withhold ZYKADIA with resumption at a reduced dose as described in Table 1 [see Dosage and Administration (2.2) and Adverse Reactions (6)]. | |||
=====Hepatotoxicity===== | |||
*Drug-induced hepatotoxicity occurred in patients treated with ZYKADIA. Elevations in alanine aminotransferase (ALT) greater than 5 times the upper limit of normal (ULN) occurred in 27% of 255 patients in Study 1. One patient (0.4%) required permanent discontinuation due to elevated transaminases and jaundice. | |||
*Monitor with liver laboratory tests including ALT, aspartate aminotransferase (AST), and total bilirubin once a month and as clinically indicated, with more frequent testing in patients who develop transaminase elevations. Based on the severity of the adverse drug reaction, withhold ZYKADIA with resumption at a reduced dose, or permanently discontinue ZYKADIA as described in Table 1 [see Dosage and Administration (2.2) and Adverse Reactions (6)]. | |||
=====Interstitial Lung Disease (ILD)/Pneumonitis===== | |||
Severe, life-threatening, or fatal ILD/pneumonitis can occur in patients treated with ZYKADIA. In Study 1, pneumonitis was reported in 4% of 255 patients treated with ZYKADIA. CTCAE Grade 3 or 4 ILD/pneumonitis was reported in 3% of patients, and fatal ILD/pneumonitis was reported in 1 patient (0.4%) in Study 1. One percent (1%) of patients discontinued ZYKADIA in Study 1 due to ILD/pneumonitis. | |||
Monitor patients for pulmonary symptoms indicative of ILD/pneumonitis. Exclude other potential causes of ILD/pneumonitis, and permanently discontinue ZYKADIA in patients diagnosed with treatment-related ILD/pneumonitis [see Dosage and Administration (2.2) and Adverse Reactions (6)]. | |||
=====QT Interval Prolongation===== | |||
*QTc interval prolongation occurs in patients treated with ZYKADIA. Three percent (3%) of 255 patients experienced a QTc interval increase over baseline greater than 60 msec in Study 1. Across the development program of ZYKADIA, one of 304 patients (less than 1%) treated with ZYKADIA doses ranging from 50 to 750 mg was found to have a QTc greater than 500 msec and 3% of patients had an increase from baseline QTc greater than 60 msec. A pharmacokinetic analysis suggested that ZYKADIA causes concentration-dependent increases in the QTc interval. | |||
*When possible, avoid use of ZYKADIA in patients with congenital long QT syndrome. Conduct periodic monitoring with electrocardiograms (ECGs) and electrolytes in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, or those who are taking medications that are known to prolong the QTc interval. Withhold ZYKADIA in patients who develop a QTc interval greater than 500 msec on at least 2 separate ECGs until the QTc interval is less than 481 msec or recovery to baseline if the QTc interval is greater than or equal to 481 msec, then resume ZYKADIA at a reduced dose as described in Table 1. Permanently discontinue ZYKADIA in patients who develop QTc interval prolongation in combination with Torsade de pointes or polymorphic ventricular tachycardia or signs/symptoms of serious arrhythmia [see Dosage and Administration (2.2) and Clinical Pharmacology (12.2)]. | |||
===== | =====Hyperglycemia===== | ||
*Hyperglycemia can occur in patients receiving ZYKADIA. In Study 1, CTCAE Grade 3–4 hyperglycemia, based on laboratory values, occurred in 13% of 255 patients. There was a 6-fold increase in the risk of CTCAE Grade 3–4 hyperglycemia in patients with diabetes or glucose intolerance and a 2-fold increase in patients taking corticosteroids. | |||
*Monitor serum glucose levels as clinically indicated. Initiate or optimize anti-hyperglycemic medications as indicated. Based on the severity of the adverse drug reaction, withhold ZYKADIA until hyperglycemia is adequately controlled, then resume ZYKADIA at a reduced dose as described in Table 1. If adequate hyperglycemic control cannot be achieved with optimal medical management, permanently discontinue ZYKADIA [see Dosage and Administration (2.2) and Adverse Reactions (6)]. | |||
=====Bradycardia===== | |||
*Bradycardia can occur in patients receiving ZYKADIA. In Study 1, sinus bradycardia, defined as a heart rate of less than 50 beats per minute, was noted as a new finding in 1% of 255 patients. Bradycardia was reported as an adverse drug reaction in 3% of patients in Study 1. | |||
*Avoid using ZYKADIA in combination with other agents known to cause bradycardia (e.g., beta-blockers, non-dihydropyridine calcium channel blockers, clonidine, and digoxin) to the extent possible. Monitor heart rate and blood pressure regularly. In cases of symptomatic bradycardia that is not life-threatening, withhold ZYKADIA until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, evaluate the use of concomitant medications, and adjust the dose of ZYKADIA. Permanently discontinue ZYKADIA for life-threatening bradycardia if no contributing concomitant medication is identified; however, if associated with a concomitant medication known to cause bradycardia or hypotension, withhold ZYKADIA until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, and if the concomitant medication can be adjusted or discontinued, resume ZYKADIA at a reduced dose as described in Table 1 upon recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, with frequent monitoring [see Dosage and Administration (2.2) and Adverse Reactions (6)]. | |||
=====Embryofetal Toxicity===== | |||
*Based on its mechanism of action, ZYKADIA may cause fetal harm when administered to a pregnant woman. In animal studies, administration of ceritinib to rats and rabbits during organogenesis at maternal plasma exposures below the recommended human dose of 750 mg daily caused increases in skeletal anomalies in rats and rabbits. Apprise women of reproductive potential of the potential hazard to a fetus [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with ZYKADIA and for at least 2 weeks following completion of therapy | |||
====Precautions==== | |||
* Description | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
===== | *The following adverse reactions are discussed in greater detail in other sections of the labeling: | ||
:*Severe or Persistent Gastrointestinal Toxicity [see Warnings and Precautions (5.1)] | |||
:*Hepatotoxicity [see Warnings and Precautions (5.2)] | |||
:*Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.3)] | |||
:*QT Interval Prolongation [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.2)] | |||
:*Hyperglycemia [see Warnings and Precautions (5.5)] | |||
:*Bradycardia [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.2)] | |||
=====Clinical Trials Experience===== | |||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
*The safety evaluation of ZYKADIA is based on 255 ALK-positive patients in Study 1 (246 patients with NSCLC and 9 patients with other cancers who received ZYKADIA at a dose of 750 mg daily). The median duration of exposure to ZYKADIA was 6 months. The study population characteristics were: median age 53 years, age less than 65 (84%), female (53%), Caucasian (63%), Asian (34%), NSCLC adenocarcinoma histology (90%), never or former smoker (97%), ECOG PS 0 or 1 (89%), brain metastasis (49%), and number of prior therapies 2 or more (67%). | |||
*Dose reductions due to adverse reactions occurred in 59% of patients treated with ZYKADIA. The most frequent adverse reactions, reported in at least 10% of patients, that led to dose reductions or interruptions were: increased ALT (29%), nausea (20%), increased AST (16%), diarrhea (16%), and vomiting (16%). Serious adverse drug reactions reported in 2% or more of patients in Study 1 were convulsion, pneumonia, ILD/pneumonitis, dyspnea, dehydration, hyperglycemia, and nausea. Fatal adverse reactions in patients treated with ZYKADIA occurred in 5% of patients, consisting of: pneumonia (4 patients), respiratory failure, ILD/pneumonitis, pneumothorax, gastric hemorrhage, general physical health deterioration, pulmonary tuberculosis, cardiac tamponade, and sepsis (1 patient each). Discontinuation of therapy due to adverse reactions occurred in 10% of patients treated with ZYKADIA. The most frequent adverse drug reactions that led to discontinuation in 1% or more of patients in Study 1 were pneumonia, ILD/pneumonitis, and decreased appetite. | |||
*Tables 2 and 3 summarize the common adverse reactions and laboratory abnormalities observed in ZYKADIA-treated patients. | |||
TABLE03 | |||
*Additional clinically significant adverse reactions occurring in 2% or more of patients treated with ZYKADIA included neuropathy (17%; comprised of paresthesia, muscular weakness, gait disturbance, peripheral neuropathy, hypoesthesia, peripheral sensory neuropathy, dysesthesia, neuralgia, peripheral motor neuropathy, hypotonia, or polyneuropathy), vision disorder (9%; comprised of vision impairment, blurred vision, photopsia, accommodation disorder, presbyopia, or reduced visual acuity), prolonged QT interval (4%), and bradycardia (3%). | |||
TABLE04 | |||
| Line 307: | Line 307: | ||
|drugInteractions= | |drugInteractions= | ||
* | * Effect of Other Drugs on Ceritinib | ||
Ceritinib is primarily metabolized by CYP3A4 and is a substrate of the efflux transporter P-glycoprotein (P-gp). | |||
Strong CYP3A Inhibitors | |||
Ketoconazole (a strong CYP3A4/P-gp inhibitor) increased the systemic exposure of ceritinib [see Clinical Pharmacology (12.3)]. Avoid concurrent use of strong CYP3A inhibitors during treatment with ZYKADIA. If concomitant use of strong CYP3A inhibitors including certain antivirals (e.g., ritonavir), macrolide antibiotics (e.g., telithromycin), antifungals (e.g., ketoconazole), and nefazodone is unavoidable, reduce the ZYKADIA dose by approximately one-third, rounded to the nearest 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the ZYKADIA dose that was taken prior to initiating the strong CYP3A4 inhibitor. | |||
Do not consume grapefruit and grapefruit juice as they may inhibit CYP3A. | |||
Strong CYP3A Inducers | |||
Rifampin (a strong CYP3A4/P-gp inducer) decreased the systemic exposure of ceritinib [see Clinical Pharmacology (12.3)]. Avoid concurrent use of strong CYP3A inducers (e.g., carbamazepine, phenytoin, rifampin, and St. John’s Wort) during treatment with ZYKADIA. | |||
7.2 Effect of Ceritinib on Other Drugs | |||
<!--Use in Specific Populations--> | Ceritinib may inhibit CYP3A and CYP2C9 at clinical concentrations [see Clinical Pharmacology (12.3)]. Avoid concurrent use of CYP3A and CYP2C9 substrates known to have narrow therapeutic indices or substrates primarily metabolized by CYP3A and CYP2C9 during treatment with ZYKADIA. If use of these medications is unavoidable, consider dose reduction of CYP3A substrates with narrow therapeutic indices (e.g., alfentanil, cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus) and CYP2C9 substrates with narrow therapeutic indices (e.g., phenytoin, warfarin).<!--Use in Specific Populations--> | ||
|useInPregnancyFDA= | |useInPregnancyFDA= | ||
Revision as of 19:39, 6 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Ceritinib is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Non-small cell lung cancer
- ZYKADIA is indicated for the treatment of patients with anaplastic lymphoma kinase (ALK)-positive metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib.
- This indication is approved under accelerated approval based on tumor response rate and duration of response [see Clinical Studies (14)]. An improvement in survival or disease-related symptoms has not been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Dosing and Administration
- The recommended dose of ZYKADIA is 750 mg orally once daily until disease progression or unacceptable toxicity. Administer ZYKADIA on an empty stomach (i.e., do not administer within 2 hours of a meal) [see Clinical Pharmacology (12.3)].
- A recommended dose has not been determined for patients with moderate to severe hepatic impairment [see Use in Specific Populations (8.6)].
- If a dose of ZYKADIA is missed, make up that dose unless the next dose is due within 12 hours.
Dose Modifications for Adverse Reactions
- Recommendations for dose modifications of ZYKADIA for adverse reactions are provided in Table 1.
- Approximately 60% of patients initiating treatment at the recommended dose required at least one dose reduction and the median time to first dose reduction was 7 weeks.
- Discontinue ZYKADIA for patients unable to tolerate 300 mg daily.
TABLE02
Dose Modification for Strong CYP3A4 Inhibitors
- Avoid concurrent use of strong CYP3A inhibitors during treatment with ZYKADIA [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
- If concomitant use of a strong CYP3A inhibitor is unavoidable, reduce the ZYKADIA dose by approximately one-third, rounded to the nearest 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the ZYKADIA dose that was taken prior to initiating the strong CYP3A4 inhibitor.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Ceritinib in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceritinib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Ceritinib in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Ceritinib in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceritinib in pediatric patients.
Contraindications
- None
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Severe or Persistent Gastrointestinal Toxicity
- Diarrhea, nausea, vomiting, or abdominal pain occurred in 96% of 255 patients including severe cases in 14% of patients treated with ZYKADIA in Study 1. Dose modification due to diarrhea, nausea, vomiting, or abdominal pain occurred in 38% of patients.
- Monitor and manage patients using standards of care, including anti-diarrheals, anti-emetics, or fluid replacement, as indicated. Based on the severity of the adverse drug reaction, withhold ZYKADIA with resumption at a reduced dose as described in Table 1 [see Dosage and Administration (2.2) and Adverse Reactions (6)].
Hepatotoxicity
- Drug-induced hepatotoxicity occurred in patients treated with ZYKADIA. Elevations in alanine aminotransferase (ALT) greater than 5 times the upper limit of normal (ULN) occurred in 27% of 255 patients in Study 1. One patient (0.4%) required permanent discontinuation due to elevated transaminases and jaundice.
- Monitor with liver laboratory tests including ALT, aspartate aminotransferase (AST), and total bilirubin once a month and as clinically indicated, with more frequent testing in patients who develop transaminase elevations. Based on the severity of the adverse drug reaction, withhold ZYKADIA with resumption at a reduced dose, or permanently discontinue ZYKADIA as described in Table 1 [see Dosage and Administration (2.2) and Adverse Reactions (6)].
Interstitial Lung Disease (ILD)/Pneumonitis
Severe, life-threatening, or fatal ILD/pneumonitis can occur in patients treated with ZYKADIA. In Study 1, pneumonitis was reported in 4% of 255 patients treated with ZYKADIA. CTCAE Grade 3 or 4 ILD/pneumonitis was reported in 3% of patients, and fatal ILD/pneumonitis was reported in 1 patient (0.4%) in Study 1. One percent (1%) of patients discontinued ZYKADIA in Study 1 due to ILD/pneumonitis.
Monitor patients for pulmonary symptoms indicative of ILD/pneumonitis. Exclude other potential causes of ILD/pneumonitis, and permanently discontinue ZYKADIA in patients diagnosed with treatment-related ILD/pneumonitis [see Dosage and Administration (2.2) and Adverse Reactions (6)].
QT Interval Prolongation
- QTc interval prolongation occurs in patients treated with ZYKADIA. Three percent (3%) of 255 patients experienced a QTc interval increase over baseline greater than 60 msec in Study 1. Across the development program of ZYKADIA, one of 304 patients (less than 1%) treated with ZYKADIA doses ranging from 50 to 750 mg was found to have a QTc greater than 500 msec and 3% of patients had an increase from baseline QTc greater than 60 msec. A pharmacokinetic analysis suggested that ZYKADIA causes concentration-dependent increases in the QTc interval.
- When possible, avoid use of ZYKADIA in patients with congenital long QT syndrome. Conduct periodic monitoring with electrocardiograms (ECGs) and electrolytes in patients with congestive heart failure, bradyarrhythmias, electrolyte abnormalities, or those who are taking medications that are known to prolong the QTc interval. Withhold ZYKADIA in patients who develop a QTc interval greater than 500 msec on at least 2 separate ECGs until the QTc interval is less than 481 msec or recovery to baseline if the QTc interval is greater than or equal to 481 msec, then resume ZYKADIA at a reduced dose as described in Table 1. Permanently discontinue ZYKADIA in patients who develop QTc interval prolongation in combination with Torsade de pointes or polymorphic ventricular tachycardia or signs/symptoms of serious arrhythmia [see Dosage and Administration (2.2) and Clinical Pharmacology (12.2)].
Hyperglycemia
- Hyperglycemia can occur in patients receiving ZYKADIA. In Study 1, CTCAE Grade 3–4 hyperglycemia, based on laboratory values, occurred in 13% of 255 patients. There was a 6-fold increase in the risk of CTCAE Grade 3–4 hyperglycemia in patients with diabetes or glucose intolerance and a 2-fold increase in patients taking corticosteroids.
- Monitor serum glucose levels as clinically indicated. Initiate or optimize anti-hyperglycemic medications as indicated. Based on the severity of the adverse drug reaction, withhold ZYKADIA until hyperglycemia is adequately controlled, then resume ZYKADIA at a reduced dose as described in Table 1. If adequate hyperglycemic control cannot be achieved with optimal medical management, permanently discontinue ZYKADIA [see Dosage and Administration (2.2) and Adverse Reactions (6)].
Bradycardia
- Bradycardia can occur in patients receiving ZYKADIA. In Study 1, sinus bradycardia, defined as a heart rate of less than 50 beats per minute, was noted as a new finding in 1% of 255 patients. Bradycardia was reported as an adverse drug reaction in 3% of patients in Study 1.
- Avoid using ZYKADIA in combination with other agents known to cause bradycardia (e.g., beta-blockers, non-dihydropyridine calcium channel blockers, clonidine, and digoxin) to the extent possible. Monitor heart rate and blood pressure regularly. In cases of symptomatic bradycardia that is not life-threatening, withhold ZYKADIA until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, evaluate the use of concomitant medications, and adjust the dose of ZYKADIA. Permanently discontinue ZYKADIA for life-threatening bradycardia if no contributing concomitant medication is identified; however, if associated with a concomitant medication known to cause bradycardia or hypotension, withhold ZYKADIA until recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, and if the concomitant medication can be adjusted or discontinued, resume ZYKADIA at a reduced dose as described in Table 1 upon recovery to asymptomatic bradycardia or to a heart rate of 60 bpm or above, with frequent monitoring [see Dosage and Administration (2.2) and Adverse Reactions (6)].
Embryofetal Toxicity
- Based on its mechanism of action, ZYKADIA may cause fetal harm when administered to a pregnant woman. In animal studies, administration of ceritinib to rats and rabbits during organogenesis at maternal plasma exposures below the recommended human dose of 750 mg daily caused increases in skeletal anomalies in rats and rabbits. Apprise women of reproductive potential of the potential hazard to a fetus [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with ZYKADIA and for at least 2 weeks following completion of therapy
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Severe or Persistent Gastrointestinal Toxicity [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Interstitial Lung Disease/Pneumonitis [see Warnings and Precautions (5.3)]
- QT Interval Prolongation [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.2)]
- Hyperglycemia [see Warnings and Precautions (5.5)]
- Bradycardia [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.2)]
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- The safety evaluation of ZYKADIA is based on 255 ALK-positive patients in Study 1 (246 patients with NSCLC and 9 patients with other cancers who received ZYKADIA at a dose of 750 mg daily). The median duration of exposure to ZYKADIA was 6 months. The study population characteristics were: median age 53 years, age less than 65 (84%), female (53%), Caucasian (63%), Asian (34%), NSCLC adenocarcinoma histology (90%), never or former smoker (97%), ECOG PS 0 or 1 (89%), brain metastasis (49%), and number of prior therapies 2 or more (67%).
- Dose reductions due to adverse reactions occurred in 59% of patients treated with ZYKADIA. The most frequent adverse reactions, reported in at least 10% of patients, that led to dose reductions or interruptions were: increased ALT (29%), nausea (20%), increased AST (16%), diarrhea (16%), and vomiting (16%). Serious adverse drug reactions reported in 2% or more of patients in Study 1 were convulsion, pneumonia, ILD/pneumonitis, dyspnea, dehydration, hyperglycemia, and nausea. Fatal adverse reactions in patients treated with ZYKADIA occurred in 5% of patients, consisting of: pneumonia (4 patients), respiratory failure, ILD/pneumonitis, pneumothorax, gastric hemorrhage, general physical health deterioration, pulmonary tuberculosis, cardiac tamponade, and sepsis (1 patient each). Discontinuation of therapy due to adverse reactions occurred in 10% of patients treated with ZYKADIA. The most frequent adverse drug reactions that led to discontinuation in 1% or more of patients in Study 1 were pneumonia, ILD/pneumonitis, and decreased appetite.
- Tables 2 and 3 summarize the common adverse reactions and laboratory abnormalities observed in ZYKADIA-treated patients.
TABLE03
- Additional clinically significant adverse reactions occurring in 2% or more of patients treated with ZYKADIA included neuropathy (17%; comprised of paresthesia, muscular weakness, gait disturbance, peripheral neuropathy, hypoesthesia, peripheral sensory neuropathy, dysesthesia, neuralgia, peripheral motor neuropathy, hypotonia, or polyneuropathy), vision disorder (9%; comprised of vision impairment, blurred vision, photopsia, accommodation disorder, presbyopia, or reduced visual acuity), prolonged QT interval (4%), and bradycardia (3%).
TABLE04
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Ceritinib in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Effect of Other Drugs on Ceritinib
Ceritinib is primarily metabolized by CYP3A4 and is a substrate of the efflux transporter P-glycoprotein (P-gp).
Strong CYP3A Inhibitors
Ketoconazole (a strong CYP3A4/P-gp inhibitor) increased the systemic exposure of ceritinib [see Clinical Pharmacology (12.3)]. Avoid concurrent use of strong CYP3A inhibitors during treatment with ZYKADIA. If concomitant use of strong CYP3A inhibitors including certain antivirals (e.g., ritonavir), macrolide antibiotics (e.g., telithromycin), antifungals (e.g., ketoconazole), and nefazodone is unavoidable, reduce the ZYKADIA dose by approximately one-third, rounded to the nearest 150 mg dosage strength. After discontinuation of a strong CYP3A inhibitor, resume the ZYKADIA dose that was taken prior to initiating the strong CYP3A4 inhibitor.
Do not consume grapefruit and grapefruit juice as they may inhibit CYP3A.
Strong CYP3A Inducers
Rifampin (a strong CYP3A4/P-gp inducer) decreased the systemic exposure of ceritinib [see Clinical Pharmacology (12.3)]. Avoid concurrent use of strong CYP3A inducers (e.g., carbamazepine, phenytoin, rifampin, and St. John’s Wort) during treatment with ZYKADIA.
7.2 Effect of Ceritinib on Other Drugs
Ceritinib may inhibit CYP3A and CYP2C9 at clinical concentrations [see Clinical Pharmacology (12.3)]. Avoid concurrent use of CYP3A and CYP2C9 substrates known to have narrow therapeutic indices or substrates primarily metabolized by CYP3A and CYP2C9 during treatment with ZYKADIA. If use of these medications is unavoidable, consider dose reduction of CYP3A substrates with narrow therapeutic indices (e.g., alfentanil, cyclosporine, dihydroergotamine, ergotamine, fentanyl, pimozide, quinidine, sirolimus, tacrolimus) and CYP2C9 substrates with narrow therapeutic indices (e.g., phenytoin, warfarin).
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ceritinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ceritinib during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Ceritinib with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Ceritinib with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Ceritinib with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Ceritinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ceritinib with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Ceritinib in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Ceritinib in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ceritinib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ceritinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Ceritinib in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Ceritinib in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Ceritinib in the drug label.
Pharmacology
There is limited information regarding Ceritinib Pharmacology in the drug label.
Mechanism of Action
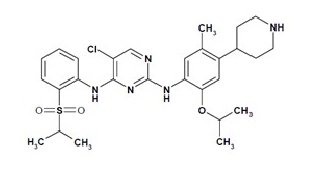
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Ceritinib in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Ceritinib in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Ceritinib in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Ceritinib in the drug label.
How Supplied
Storage
There is limited information regarding Ceritinib Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Ceritinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Ceritinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Ceritinib in the drug label.
Precautions with Alcohol
- Alcohol-Ceritinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Ceritinib |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Ceritinib |Label Name=Ceritinib11.png
}}
{{#subobject:
|Label Page=Ceritinib |Label Name=Ceritinib11.png
}}