|
|
| Line 1: |
Line 1: |
| __NOTOC__ | | __NOTOC__ |
| | |
| '''For patient information click [[Cardiomyopathy (patient information)|here]]''' | | '''For patient information click [[Cardiomyopathy (patient information)|here]]''' |
| {{Infobox_Disease | | | {{Infobox_Disease | |
| Line 14: |
Line 15: |
| == [[Cardiomyopathy overview|Overview]] == | | == [[Cardiomyopathy overview|Overview]] == |
| ==[[ Cardiomyopathy historical perspective| Historical Perspective]]== | | ==[[ Cardiomyopathy historical perspective| Historical Perspective]]== |
|
| |
| * In 1980, the World Health Organization (WHO) defined cardiomyopathies as "heart muscle diseases of unknown cause" to distinguish cardiomyopathy from cardiac dysfunction due to known cardiovascular causes such as hypertension, ischemic heart disease, or valvular disease. In clinical practice, however, the term "cardiomyopathy" had also been applied to diseases of known cardiovascular cause, including ischemic cardiomyopathy and hypertensive cardiomyopathy.
| |
|
| |
| * As a result, the 1995 WHO/International Society and Federation of Cardiology (ISFC) Task Force on the Definition and Classification of the Cardiomyopathies expanded the classification to include all diseases affecting heart muscle and to take into consideration etiology as well as the dominant pathophysiology. In the 1995 classification, the cardiomyopathies were defined as "diseases of the myocardium associated with cardiac dysfunction." They were classified according to anatomy and physiology into the following types:
| |
|
| |
| ●Dilated cardiomyopathy (DCM)
| |
|
| |
| ●Hypertrophic cardiomyopathy (HCM)
| |
|
| |
| ●Restrictive cardiomyopathy (RCM)
| |
|
| |
| ●Arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D)
| |
|
| |
| ●Unclassified cardiomyopathies
| |
|
| |
| * Then, a 2006 AHA scientific statement proposed a contemporary definition and classification of the cardiomyopathies: "Cardiomyopathies are a heterogeneous group of diseases of the myocardium associated with mechanical and/or electrical dysfunction that usually (but not invariably) exhibit inappropriate ventricular hypertrophy or dilation and are due to a variety of causes that frequently are genetic. Cardiomyopathies either are confined to the heart or are a part of generalized systemic disorders, often leading to cardiovascular death or progressive heart failure-related disability."
| |
| As such, cardiomyopathies are categorized into two groups:
| |
|
| |
| 1- Primary cardiomyopathies (predominantly involving the heart): The primary cardiomyopathies are subdivided into those which are genetic, mixed (predominantly nongenetic; less commonly genetic), or acquired.
| |
| A- The genetic cardiomyopathies include HCM, ARVC/D, left ventricular noncompaction, PRKAG2 and Danon glycogen storage diseases, conduction defects, mitochondrial myopathies, and ion channel disorders.
| |
| B- The mixed cardiomyopathies include DCM and RCM.
| |
| C- The acquired cardiomyopathies include myocarditis, stress-induced (takotsubo), peripartum and tachycardia-induced.
| |
|
| |
| 2- Secondary cardiomyopathies (accompanied by other organ system involvement).
| |
|
| |
| * Then, in 2008, the ESC working group on myocardial and pericardial diseases presented an update to the WHO/ISFC classification in which cardiomyopathy was defined as: "A myocardial disorder in which the heart muscle is structurally and functionally abnormal in the absence of coronary artery disease, hypertension, valvular disease and congenital heart disease sufficient to explain the observed myocardial abnormality".
| |
|
| |
| * Despite that, the term "cardiomyopathy" continues to be used in patients with ischemic, hypertensive, valvular and congenital heart diseases.
| |
|
| |
| == [[Cardiomyopathy classification|Classification]] ==
| |
|
| |
| * In clinical practice, cardiomyopathy can be classified into two main groups based on the main etiology: ischemic versus non-ischemic.
| |
|
| |
| * Moreover, cardiomyopathy has been also classified into two main groups, based on the presence of systolic and/or diastolic function: Heart failure with reduced ejection fraction (HFrEF) versus heart failure with preserved ejection fraction (HFpEF). Most experts agree that ejection fraction of less than 40 should be classified as HFrEF; on the other hand, those patients with EF of more than 40 should be classified as HFpEF.
| |
|
| |
| * Classification of HF severity — The history, including assessment of New York Heart Association (NYHA) functional class, and physical examination in conjunction with the diagnostic tests reviewed below should both establish the primary cause of the HF and provide a reasonable estimate of its severity.
| |
|
| |
| * The classification system that is most commonly used is the NYHA classification: which classifies patients into 4 categories based on their functional limitation:
| |
|
| |
| ●Class I – Patients with heart disease without resulting limitation of physical activity. Ordinary physical activity does not cause HF symptoms such as fatigue or dyspnea.
| |
|
| |
| ●Class II – Patients with heart disease resulting in slight limitation of physical activity. Symptoms of HF develop with ordinary activity but there are no symptoms at rest.
| |
|
| |
| ●Class III – Patients with heart disease resulting in marked limitation of physical activity. Symptoms of HF develop with less than ordinary physical activity but there are no symptoms at rest.
| |
|
| |
| ●Class IV – Patients with heart disease resulting in inability to carry on any physical activity without discomfort. Symptoms of HF may occur even at rest.
| |
|
| |
|
| |
| * Another commonly-used classification of the stages of HF, as outlined by the American College of Cardiology Foundation/American Heart Association guidelines:
| |
|
| |
| ●Stage A – At high risk for HF but without structural heart disease or symptoms of HF.
| |
|
| |
| ●Stage B – Structural heart disease but without signs or symptoms of HF. This stage includes patients in NYHA functional class I with no prior or current symptoms or signs of HF.
| |
|
| |
| ●Stage C – Structural heart disease with prior or current symptoms of HF. This stage includes patients in any NYHA functional class (including class I with prior symptoms).
| |
|
| |
| ●Stage D – Refractory HF requiring specialized interventions. This stage includes patients in NYHA functional class IV with refractory HF.
| |
|
| |
| == [[Cardiomyopathy pathophysiology|Pathophysiology]] ==
| |
|
| |
| * Different causes of cardiomyopathies result in abnormal myocardial function and structure, leading to the development of clinical heart failure symptoms, early or later in life, based on the cause, genetic expression and penetrance, and presence of environmental factors.
| |
|
| |
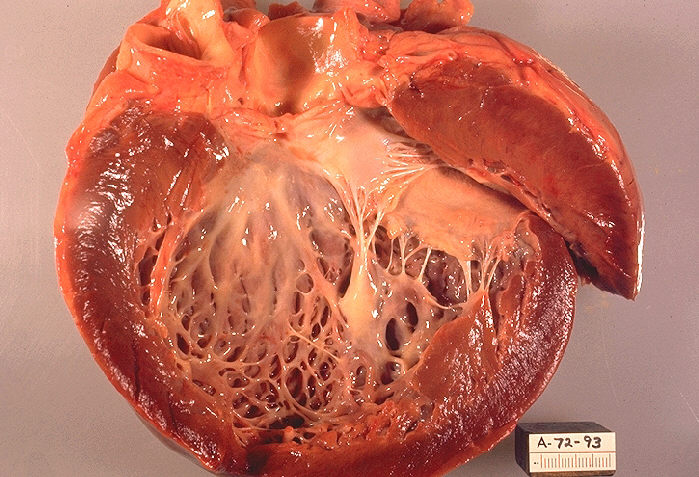
| * Dilated cardiomyopathy (DCM) is characterized by dilation and impaired contraction of one or both ventricles. The dilation often becomes severe and is invariably accompanied by an increase in total cardiac mass. Affected patients have impaired systolic function and clinical presentation is usually with features of heart failure (HF).
| |
|
| |
| * In approximately 60-70% of patients, HCM is caused by mutations in sarcomeric contractile protein genes and is transmitted as an autosomal dominant trait with incomplete penetrance. The most common mutations are in the beta myosin heavy chain and the cardiac myosin-binding protein C genes. Characteristic histologic changes include myocyte hypertrophy and disarray, which usually corresponds to the areas of greatest hypertrophy. The LV volume is normal or reduced, and diastolic dysfunction is usually present. Systolic pressure gradients in the left ventricular outflow tract during resting conditions are found in approximately one-quarter of patients.
| |
|
| |
| * Restrictive cardiomyopathy (RCM) is characterized by nondilated ventricles with impaired ventricular filling.
| |
|
| |
| == [[Cardiomyopathy causes|Causes]] ==
| |
|
| |
| * Based on AHA2006 classification of cardiomyopathies into primary and secondary groups based on the involvement of other organ system, causes can be summarized as follows:
| |
|
| |
| 1- Primary cardiomyopathies (predominantly involving the heart): The primary cardiomyopathies are subdivided into those which are genetic, mixed (predominantly nongenetic; less commonly genetic), or acquired.
| |
| A- The genetic cardiomyopathies include HCM, ARVC/D, left ventricular noncompaction, PRKAG2 and Danon glycogen storage diseases, conduction defects, mitochondrial myopathies, and ion channel disorders.
| |
| B- The mixed cardiomyopathies include DCM and RCM.
| |
| C- The acquired cardiomyopathies include myocarditis, stress-induced (takotsubo), peripartum and tachycardia-induced.
| |
|
| |
| 2- Secondary cardiomyopathies (accompanied by other organ system involvement).
| |
|
| |
| * Endomyocardial fibrosis (EMF) occurs mainly in children and adolescents in the tropics. The cause is unknown, but proposed contributing factors include infection, environmental exposure, immunologic processes, and genetics. In some patients, it is associated with severe hypereosinophilia in the early stages of the illness. Disease progression includes endocardial fibrosis and thrombosis, particularly affecting the apical ventricles and subvalvular apparatus. In the later stages, restrictive physiology is prominent, with HF and valve regurgitation.
| |
|
| |
| * Arrhythmogenic right ventricular cardiomyopathy is a genetically determined heart muscle disease characterized by ventricular arrhythmias and a specific myocardial pathology. The myocardium of the right ventricular free wall (and frequently the LV as well) is replaced by fibrous and/or fibro-fatty tissue, with scattered residual myocardial cells. Right ventricular function is abnormal, with regional akinesis or dyskinesis and, in severe cases, there is global right ventricular dilation and dysfunction. Mutations in desmosomal genes cause disease in 40-60% of cases, and also cause arrhythmogenic left ventricular cardiomyopathy with similar arrhythmic and pathological manifestations.
| |
|
| |
| * Left ventricular noncompaction — LV noncompaction, also called isolated ventricular noncompaction, is a rare unclassified cardiomyopathy with an altered myocardial wall due to intrauterine arrest of compaction of the loose interwoven meshwork. There is continuity between the LV cavity and deep intratrabecular recesses that are filled with blood from the ventricular cavity without evidence of communication to the epicardial arterial system. When the morphological changes are severe, LV noncompaction may be associated with HF, thromboembolism, and ventricular arrhythmias in adults.
| |
|
| |
| * Stress-induced cardiomyopathy — Stress-induced cardiomyopathy, also called apical ballooning syndrome, broken heart syndrome, and takotsubo cardiomyopathy, is an increasingly reported syndrome generally characterized by transient systolic dysfunction of the apical and/or mid segments of the LV that is often provoked by stress.
| |
|
| |
| == [[Cardiomyopathy differential diagnosis|Differentiating Cardiomyopathy from other Diseases]] ==
| |
|
| |
| * It is important to differentiate cardiomyopathy from other causes of myocardial hypertrophy, including athlete heart.
| |
|
| |
| == [[Cardiomyopathy epidemiology and demographics|Epidemiology and Demographics]] == | | == [[Cardiomyopathy epidemiology and demographics|Epidemiology and Demographics]] == |
|
| |
| * Incidence and prevalence differ based on cause.
| |
|
| |
| * The incidence of DCM has been estimated to be five to eight cases per 100,000 population, with a prevalence of 36 per 100,000. This could be explained by the incomplete disease expression, which goes unrecognized. It has been suggested that up to 14 percent of the middle-aged and elderly population have asymptomatic left ventricular (LV) systolic dysfunction.
| |
|
| |
| * The prevalence of HCM in the absence of aortic valve disease or systemic hypertension is at least 1:500 of the adult population.
| |
|
| |
| * RCM is much less common than either DCM or HCM outside the tropics, but is a frequent cause of death in Africa, India, South and Central America, and Asia, primarily because of the high incidence of endomyocardial fibrosis in those regions.
| |
|
| |
|
| ==[[Cardiomyopathy risk factors | Risk Factors]]== | | ==[[Cardiomyopathy risk factors | Risk Factors]]== |
| Line 121: |
Line 23: |
| == Diagnosis == | | == Diagnosis == |
| [[Cardiomyopathy history and symptoms|History and Symptoms]] | [[Cardiomyopathy physical examination|Physical Examination]] | [[Cardiomyopathy laboratory findings|Laboratory Findings]] | [[Cardiomyopathy electrocardiogram|Electrocardiogram]] | [[Cardiomyopathy chest x ray | Chest X Ray]] | [[Cardiomyopathy CT| CT]] | [[Cardiomyopathy MRI|MRI]] | [[Cardiomyopathy echocardiography or ultrasound|Echocardiography]] | [[Cardiomyopathy other imaging findings| Other Imaging Findings]] | [[Cardiomyopathy other diagnostic studies|Other Diagnostic Studies]] | | [[Cardiomyopathy history and symptoms|History and Symptoms]] | [[Cardiomyopathy physical examination|Physical Examination]] | [[Cardiomyopathy laboratory findings|Laboratory Findings]] | [[Cardiomyopathy electrocardiogram|Electrocardiogram]] | [[Cardiomyopathy chest x ray | Chest X Ray]] | [[Cardiomyopathy CT| CT]] | [[Cardiomyopathy MRI|MRI]] | [[Cardiomyopathy echocardiography or ultrasound|Echocardiography]] | [[Cardiomyopathy other imaging findings| Other Imaging Findings]] | [[Cardiomyopathy other diagnostic studies|Other Diagnostic Studies]] |
|
| |
| * Identification of various cardiomyopathy phenotypes relies primarily upon echocardiographic evaluation.
| |
|
| |
| * In select cases, advanced cardiac imaging, using cardiac magnetic resonance imaging or computed tomography, may be useful to identify and localize fatty infiltration, inflammation, scar/fibrosis, focal hypertrophy, and better visualize the left ventricular apex and right ventricle.
| |
|
| |
|
| == Treatment == | | == Treatment == |