Canagliflozin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Stefano Giannoni [2],Alberto Plate [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Canagliflozin is an sodium-glucose co-transporter 2 (SGLT2) inhibitor that is FDA approved for the treatment of type 2 diabetes mellitus. It is used as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.. Common adverse reactions include female genital mycotic infections, urinary tract infection, and increased urination.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Canagliflozin FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Canagliflozin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Canagliflozin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Canagliflozin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Canagliflozin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Canagliflozin in pediatric patients.
Contraindications
- History of a serious hypersensitivity reaction to canagliflozin
- Severe renal impairment (eGFR less than 30 mL/min/1.73 m2), end stage renal disease or patients on dialysis
Warnings
Hypotension
- Canagliflozin causes intravascular volume contraction.
- Symptomatic hypotension can occur after initiating canagliflozin particularly in patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2), elderly patients, patients on either diuretics or medications that interfere with the renin-angiotensin-aldosterone system (e.g., angiotensin-converting-enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs] ), or patients with low systolic blood pressure.
- Before initiating canagliflozin in patients with one or more of these characteristics, volume status should be assessed and corrected.
- Monitor for signs and symptoms after initiating therapy.
Impairment in Renal Function
- Canagliflozin increases serum creatinine and decreases eGFR. Patients with hypovolemia may be more susceptible to these changes. Renal function abnormalities can occur after initiating canagliflozin.
- More frequent renal function monitoring is recommended in patients with an eGFR below 60 mL/min/1.73 m2.
Hyperkalemia
- Canagliflozin can lead to hyperkalemia.
- Patients with moderate renal impairment who are taking medications that interfere with potassium excretion, such as potassium-sparing diuretics, or medications that interfere with the renin-angiotensin-aldosterone system are more likely to develop hyperkalemia.
- Monitor serum potassium levels periodically after initiating canagliflozin in patients with impaired renal function and in patients predisposed to hyperkalemia due to medications or other medical conditions.
Hypoglycemia with Concomitant Use with Insulin and Insulin Secretagogues
- Insulin and insulin secretagogues are known to cause hypoglycemia. Canagliflozin can increase the risk of hypoglycemia when combined with insulin or an insulin secretagogue. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with canagliflozin.
Genital Mycotic Infections
- Canagliflozin increases the risk of genital mycotic infections. Patients with a history of genital mycotic infections and uncircumcised males were more likely to develop genital mycotic infections.
- Monitor and treat appropriately.
Hypersensitivity Reactions
Hypersensitivity reactions (e.g., generalized urticaria), some serious, were reported with canagliflozin treatment; these reactions generally occurred within hours to days after initiating canagliflozin. If hypersensitivity reactions occur, discontinue use of canagliflozin; treat per standard of care and monitor until signs and symptoms resolve.
Increases in Low-Density Lipoprotein (LDL-C)
- Dose-related increases in LDL-C occur with canagliflozin.
- Monitor LDL-C and treat per standard of care after initiating canagliflozin.
Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with canagliflozin or any other antidiabetic drug.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Pool of Placebo-Controlled Trials
The data in Table 1 is derived from four 26-week placebo-controlled trials. In one trial canagliflozin was used as monotherapy and in three trials canagliflozin was used as add-on therapy [see CLINICAL STUDIES (14)]. These data reflect exposure of 1667 patients to canagliflozin and a mean duration of exposure to canagliflozin of 24 weeks. Patients received canagliflozin 100 mg (N=833), canagliflozin 300 mg (N=834) or placebo (N=646) once daily. The mean age of the population was 56 years and 2% were older than 75 years of age. Fifty percent (50%) of the population was male and 72% were Caucasian, 12% were Asian, and 5% were Black or African American. At baseline the population had diabetes for an average of 7.3 years, had a mean HbA1C of 8.0% and 20% had established microvascular complications of diabetes. Baseline renal function was normal or mildly impaired (mean eGFR 88 mL/min/1.73 m2).
Table 1 shows common adverse reactions associated with the use of canagliflozin. These adverse reactions were not present at baseline, occurred more commonly on canagliflozin than on placebo, and occurred in at least 2% of patients treated with either canagliflozin 100 mg or canagliflozin 300 mg.

Abdominal pain was also more commonly reported in patients taking canagliflozin 100 mg (1.8%), 300 mg (1.7%) than in patients taking placebo (0.8%).
Pool of Placebo- and Active-Controlled Trials
The occurrence of adverse reactions was also evaluated in a larger pool of patients participating in placebo- and active-controlled trials.
The data combined eight clinical trials [see CLINICAL STUDIES (14)] and reflect exposure of 6177 patients to canagliflozin. The mean duration of exposure to canagliflozin was 38 weeks with 1832 individuals exposed to canagliflozin for greater than 50 weeks. Patients received canagliflozin 100 mg (N=3092), canagliflozin 300 mg (N=3085) or comparator (N=3262) once daily. The mean age of the population was 60 years and 5% were older than 75 years of age. Fifty-eight percent (58%) of the population was male and 73% were Caucasian, 16% were Asian, and 4% were Black or African American. At baseline, the population had diabetes for an average of 11 years, had a mean HbA1C of 8.0% and 33% had established microvascular complications of diabetes. Baseline renal function was normal or mildly impaired (mean eGFR 81 mL/min/1.73 m2).
The types and frequency of common adverse reactions observed in the pool of eight clinical trials were consistent with those listed in Table 1. In this pool, canagliflozin was also associated with the adverse reactions of fatigue (1.7% with comparator, 2.2% with canagliflozin 100 mg, and 2.0% with canagliflozin 300 mg) and loss of strength or energy (i.e., asthenia) (0.6% with comparator, 0.7% with canagliflozin 100 mg, and 1.1% with canagliflozin 300 mg).
In the pool of eight clinical trials, the incidence rate of pancreatitis (acute or chronic) was 0.9, 2.7, and 0.9 per 1000 patient-years of exposure to comparator, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
In the pool of eight clinical trials with a longer mean duration of exposure to canagliflozin (68 weeks), the incidence rate of bone fracture was 14.2, 18.7, and 17.6 per 1000 patient years of exposure to comparator, canagliflozin 100 mg, and canagliflozin 300 mg, respectively. Upper extremity fractures occurred more commonly on canagliflozin than comparator.
In the pool of eight clinical trials, hypersensitivity-related adverse reactions (including erythema, rash, pruritus, urticaria, and angioedema) occurred in 3.0%, 3.8%, and 4.2% of patients receiving comparator, canagliflozin 100 mg, and canagliflozin 300 mg, respectively. Five patients experienced serious adverse reactions of hypersensitivity with canagliflozin, which included 4 patients with urticaria and 1 patient with a diffuse rash and urticaria occurring within hours of exposure to canagliflozin. Among these patients, 2 patients discontinued canagliflozin. One patient with urticaria had recurrence when canagliflozin was re-initiated.
Photosensitivity-related adverse reactions (including photosensitivity reaction, polymorphic light eruption, and sunburn) occurred in 0.1%, 0.2%, and 0.2% of patients receiving comparator, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Other adverse reactions occurring more frequently on canagliflozin than on comparator were:
Volume Depletion-Related Adverse Reactions
canagliflozin results in an osmotic diuresis, which may lead to reductions in intravascular volume. In clinical studies, treatment with canagliflozin was associated with a dose-dependent increase in the incidence of volume depletion-related adverse reactions (e.g., hypotension, postural dizziness, orthostatic hypotension, syncope, and dehydration). An increased incidence was observed in patients on the 300 mg dose. The three factors associated with the largest increase in volume depletion-related adverse reactions were the use of loop diuretics, moderate renal impairment (eGFR 30 to less than 60 mL/min/1.73 m2), and age 75 years and older (Table 2)

Impairment in Renal Function
canagliflozin is associated with a dose-dependent increase in serum creatinine and a concomitant fall in estimated GFR (Table 3). Patients with moderate renal impairment at baseline had larger mean changes.

In the pool of four placebo-controlled trials where patients had normal or mildly impaired baseline renal function, the proportion of patients who experienced at least one event of significant renal function decline, defined as an eGFR below 80 mL/min/1.73 m2 and 30% lower than baseline, was 2.1% with placebo, 2.0% with canagliflozin 100 mg, and 4.1% with canagliflozin 300 mg. At the end of treatment, 0.5% with placebo, 0.7% with canagliflozin 100 mg, and 1.4% with canagliflozin 300 mg had a significant renal function decline.
In a trial carried out in patients with moderate renal impairment with a baseline eGFR of 30 to less than 50 mL/min/1.73 m2 (mean baseline eGFR 39 mL/min/1.73 m2) [see CLINICAL STUDIES (14.3)], the proportion of patients who experienced at least one event of significant renal function decline, defined as an eGFR 30% lower than baseline, was 6.9% with placebo, 18% with canagliflozin 100 mg, and 22.5% with canagliflozin 300 mg. At the end of treatment, 4.6% with placebo, 3.4% with canagliflozin 100 mg, and 3.4% with canagliflozin 300 mg had a significant renal function decline.
In a pooled population of patients with moderate renal impairment (N=1085) with baseline eGFR of 30 to less than 60 mL/min/1.73 m2 (mean baseline eGFR 48 mL/min/1.73 m2), the overall incidence of these events was lower than in the dedicated trial but a dose-dependent increase in incident episodes of significant renal function decline compared to placebo was still observed.
Use of canagliflozin was associated with an increased incidence of renal-related adverse reactions (e.g., increased blood creatinine, decreased glomerular filtration rate, renal impairment, and acute renal failure), particularly in patients with moderate renal impairment.
In the pooled analysis of patients with moderate renal impairment, the incidence of renal-related adverse reactions was 3.7% with placebo, 8.9% with canagliflozin 100 mg, and 9.3% with canagliflozin 300 mg. Discontinuations due to renal-related adverse events occurred in 1.0% with placebo, 1.2% with canagliflozin 100 mg, and 1.6% with canagliflozin 300 mg [see WARNINGS AND PRECAUTIONS (5.2)].
Genital Mycotic Infections
In the pool of four placebo-controlled clinical trials, female genital mycotic infections (e.g., vulvovaginal mycotic infection, vulvovaginal candidiasis, and vulvovaginitis) occurred in 3.2%, 10.4%, and 11.4% of females treated with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively. Patients with a history of genital mycotic infections were more likely to develop genital mycotic infections on canagliflozin. Female patients who developed genital mycotic infections on canagliflozin were more likely to experience recurrence and require treatment with oral or topical antifungal agents and anti-microbial agents [see WARNINGS AND PRECAUTIONS (5.5)].
In the pool of four placebo-controlled clinical trials, male genital mycotic infections (e.g., candidal balanitis, balanoposthitis) occurred in 0.6%, 4.2%, and 3.7% of males treated with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively. Male genital mycotic infections occurred more commonly in uncircumcised males and in males with a prior history of balanitis or balanoposthitis. Male patients who developed genital mycotic infections on canagliflozin were more likely to experience recurrent infections (22% on canagliflozin versus none on placebo), and require treatment with oral or topical antifungal agents and anti-microbial agents than patients on comparators. In the pooled analysis of 8 controlled trials, phimosis was reported in 0.3% of uncircumcised male patients treated with canagliflozin and 0.2% required circumcision to treat the phimosis [see WARNINGS AND PRECAUTIONS (5.5)].
Hypoglycemia
In all clinical trials, hypoglycemia was defined as any event regardless of symptoms, where biochemical hypoglycemia was documented (any glucose value below or equal to 70 mg/dL). Severe hypoglycemia was defined as an event consistent with hypoglycemia where the patient required the assistance of another person to recover, lost consciousness, or experienced a seizure (regardless of whether biochemical documentation of a low glucose value was obtained). In individual clinical trials [see CLINICAL STUDIES (14)], episodes of hypoglycemia occurred at a higher rate when canagliflozin was co-administered with insulin or sulfonylureas (Table 4)

Laboratory Tests
Increases in Serum Potassium
Dose-related, transient mean increases in serum potassium were observed early after initiation of canagliflozin (i.e., within 3 weeks) in a trial of patients with moderate renal impairment [see CLINICAL STUDIES (14.3)]. In this trial, increases in serum potassium of greater than 5.4 mEq/L and 15% above baseline occurred in 16.1%, 12.4%, and 27.0% of patients treated with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively. More severe elevations (i.e., equal or greater than 6.5 mEq/L) occurred in 1.1%, 2.2%, and 2.2% of patients treated with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively. In patients with moderate renal impairment, increases in potassium were more commonly seen in those with elevated potassium at baseline and in those using medications that reduce potassium excretion, such as potassium-sparing diuretics, angiotensin-converting-enzyme inhibitors, and angiotensin-receptor blockers
Increases in Serum Magnesium
Dose-related increases in serum magnesium were observed early after initiation of canagliflozin (within 6 weeks) and remained elevated throughout treatment. In the pool of four placebo-controlled trials, the mean change in serum magnesium levels was 8.1% and 9.3% with canagliflozin 100 mg and canagliflozin 300 mg, respectively, compared to -0.6% with placebo. In a trial of patients with moderate renal impairment [see CLINICAL STUDIES (14.3)], serum magnesium levels increased by 0.2%, 9.2%, and 14.8% with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Increases in Serum Phosphate
Dose-related increases in serum phosphate levels were observed with canagliflozin. In the pool of four placebo controlled trials, the mean change in serum phosphate levels were 3.6% and 5.1% with canagliflozin 100 mg and canagliflozin 300 mg, respectively, compared to 1.5% with placebo. In a trial of patients with moderate renal impairment [see CLINICAL STUDIES (14.3)], the mean serum phosphate levels increased by 1.2%, 5.0%, and 9.3% with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively.
Increases in Low-Density Lipoprotein Cholesterol (LDL-C) and non-High-Density Lipoprotein Cholesterol (non-HDL-C)
In the pool of four placebo-controlled trials, dose-related increases in LDL-C with canagliflozin were observed. Mean changes (percent changes) from baseline in LDL-C relative to placebo were 4.4 mg/dL (4.5%) and 8.2 mg/dL (8.0%) with canagliflozin 100 mg and canagliflozin 300 mg, respectively. The mean baseline LDL-C levels were 104 to 110 mg/dL across treatment groups [see WARNINGS AND PRECAUTIONS (5.7)].
Dose-related increases in non-HDL-C with canagliflozin were observed. Mean changes (percent changes) from baseline in non-HDL-C relative to placebo were 2.1 mg/dL (1.5%) and 5.1 mg/dL (3.6%) with canagliflozin 100 mg and 300 mg, respectively. The mean baseline non-HDL-C levels were 140 to 147 mg/dL across treatment groups.
Increases in Hemoglobin
In the pool of four placebo-controlled trials, mean changes (percent changes) from baseline in hemoglobin were -0.18 g/dL (-1.1%) with placebo, 0.47 g/dL (3.5%) with canagliflozin 100 mg, and 0.51 g/dL (3.8%) with canagliflozin 300 mg. The mean baseline hemoglobin value was approximately 14.1 g/dL across treatment groups. At the end of treatment, 0.8%, 4.0%, and 2.7% of patients treated with placebo, canagliflozin 100 mg, and canagliflozin 300 mg, respectively, had hemoglobin above the upper limit of normal.
Postmarketing Experience
There is limited information regarding Canagliflozin Postmarketing Experience in the drug label.
Drug Interactions
UGT Enzyme Inducers Rifampin: Co-administration of canagliflozin with rifampin, a nonselective inducer of several UGT enzymes, including UGT1A9, UGT2B4, decreased canagliflozin area under the curve (AUC) by 51%. This decrease in exposure to canagliflozin may decrease efficacy. If an inducer of these UGTs (e.g., rifampin, phenytoin, phenobarbital, ritonavir) must be co-administered with canagliflozin (canagliflozin), consider increasing the dose to 300 mg once daily if patients are currently tolerating canagliflozin 100 mg once daily, have an eGFR greater than 60 mL/min/1.73 m2, and require additional glycemic control. Consider other antihyperglycemic therapy in patients with an eGFR of 45 to less than 60 mL/min/1.73 m2 receiving concurrent therapy with a UGT inducer and require additional glycemic control [see DOSAGE AND ADMINISTRATION (2.3) and CLINICAL PHARMACOLOGY (12.3)].
7.2 Digoxin There was an increase in the AUC and mean peak drug concentration (Cmax) of digoxin (20% and 36%, respectively) when co-administered with canagliflozin 300 mg [see CLINICAL PHARMACOLOGY (12.3)]. Patients taking canagliflozin with concomitant digoxin should be monitored appropriately.
7.3 Positive Urine Glucose Test Monitoring glycemic control with urine glucose tests is not recommended in patients taking SGLT2 inhibitors as SGLT2 inhibitors increase urinary glucose excretion and will lead to positive urine glucose tests. Use alternative methods to monitor glycemic control.
7.4 Interference with 1,5-anhydroglucitol (1,5-AG) Assay Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking SGLT2 inhibitors. Use alternative methods to monitor glycemic control.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no adequate and well-controlled studies of canagliflozin in pregnant women. Based on results from rat studies, canagliflozin may affect renal development and maturation. In a juvenile rat study, increased kidney weights and renal pelvic and tubular dilatation were evident at greater than or equal to 0.5 times clinical exposure from a 300 mg dose [see NONCLINICAL TOXICOLOGY (13.2)].
These outcomes occurred with drug exposure during periods of animal development that correspond to the late second and third trimester of human development. During pregnancy, consider appropriate alternative therapies, especially during the second and third trimesters. canagliflozin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Canagliflozin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Canagliflozin during labor and delivery.
Nursing Mothers
It is not known if canagliflozin is excreted in human milk. canagliflozin is secreted in the milk of lactating rats reaching levels 1.4 times higher than that in maternal plasma. Data in juvenile rats directly exposed to canagliflozin showed risk to the developing kidney (renal pelvic and tubular dilatations) during maturation. Since human kidney maturation occurs in utero and during the first 2 years of life when lactational exposure may occur, there may be risk to the developing human kidney. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from canagliflozin, a decision should be made whether to discontinue nursing or to discontinue canagliflozin, taking into account the importance of the drug to the mother
Pediatric Use
Safety and effectiveness of canagliflozin in pediatric patients under 18 years of age have not been established.
Geriatic Use
wo thousand thirty-four (2034) patients 65 years and older, and 345 patients 75 years and older were exposed to canagliflozin in nine clinical studies of canagliflozin [see CLINICAL STUDIES (14.3)].
Patients 65 years and older had a higher incidence of adverse reactions related to reduced intravascular volume with canagliflozin (such as hypotension, postural dizziness, orthostatic hypotension, syncope, and dehydration), particularly with the 300 mg daily dose, compared to younger patients; more prominent increase in the incidence was seen in patients who were 75 years and older. Smaller reductions in HbA1C with canagliflozin relative to placebo were seen in older (65 years and older; -0.61% with canagliflozin 100 mg and -0.74% with canagliflozin 300 mg relative to placebo) compared to younger patients (-0.72% with canagliflozin 100 mg and -0.87% with canagliflozin 300 mg relative to placebo).
Gender
There is no FDA guidance on the use of Canagliflozin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Canagliflozin with respect to specific racial populations.
Renal Impairment
The efficacy and safety of canagliflozin were evaluated in a study that included patients with moderate renal impairment (eGFR 30 to less than 50 mL/min/1.73 m2) [see CLINICAL STUDIES (14.3)]. These patients had less overall glycemic efficacy and had a higher occurrence of adverse reactions related to reduced intravascular volume, renal-related adverse reactions, and decreases in eGFR compared to patients with mild renal impairment or normal renal function (eGFR greater than or equal to 60 mL/min/1.73 m2); patients treated with canagliflozin 300 mg were more likely to experience increases in potassium [see DOSAGE AND ADMINISTRATION (2.2), WARNINGS AND PRECAUTIONS (5.1, 5.2, and 5.3), and ADVERSE REACTIONS (6.1)].
The efficacy and safety of canagliflozin have not been established in patients with severe renal impairment (eGFR less than 30 mL/min/1.73 m2), with ESRD, or receiving dialysis. canagliflozin is not expected to be effective in these patient populations
Hepatic Impairment
No dosage adjustment is necessary in patients with mild or moderate hepatic impairment. The use of canagliflozin has not been studied in patients with severe hepatic impairment and is therefore not recommended
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Canagliflozin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Canagliflozin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- periodic blood glucose monitoring and HbA1C testing
IV Compatibility
There is limited information regarding the compatibility of Canagliflozin and IV administrations.
Overdosage
There were no reports of overdose during the clinical development program of canagliflozin (canagliflozin).
In the event of an overdose, contact the Poison Control Center. It is also reasonable to employ the usual supportive measures, e.g., remove unabsorbed material from the gastrointestinal tract, employ clinical monitoring, and institute supportive treatment as dictated by the patient's clinical status. Canagliflozin was negligibly removed during a 4-hour hemodialysis session. Canagliflozin is not expected to be dialyzable by peritoneal dialysis.
Pharmacology
 | |
| Clinical data | |
|---|---|
| Trade names | canagliflozin |
| Synonyms | JNJ-28431754; TA-7284; (1S)-1,5-anhydro-1-C-[3-[[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucitol |
| AHFS/Drugs.com | canagliflozin |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 65% |
| Protein binding | 99% |
| Metabolism | Hepatic glucuronidation |
| Elimination half-life | 11.8 (10–13) hours |
| Excretion | Fecal and 33% renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C24H25FO5S |
| Molar mass | 444.52 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Mechanism of Action
- Sodium-glucose co-transporter 2 (SGLT2), expressed in the proximal renal tubules, is responsible for the majority of the reabsorption of filtered glucose from the tubular lumen.
- Canagliflozin is an inhibitor of SGLT2. By inhibiting SGLT2, canagliflozin reduces reabsorption of filtered glucose and lowers the renal threshold for glucose (RTG), and thereby increases urinary glucose excretion.
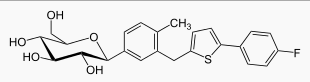
Structure
- Canagliflozin, is chemically known as (1S)-1,5-anhydro-1-[3-[5-(4-fluorophenyl)-2-thienyl]methyl]-4-methylphenyl]-D-glucitol hemihydrate and its molecular formula and weight are C24H25FO5S∙1/2 H2O and 453.53, respectively. The structural formula for canagliflozin is:

Pharmacodynamics
Following single and multiple oral doses of canagliflozin to patients with type 2 diabetes, dose-dependent decreases in the renal threshold for glucose (RTG) and increases in urinary glucose excretion were observed. From a starting value of RTG of approximately 240 mg/dL, canagliflozin at 100 mg and 300 mg once daily suppressed RTG throughout the 24-hour period. Maximal suppression of mean RTG over the 24-hour period was seen with the 300 mg daily dose to approximately 70 to 90 mg/dL in patients with type 2 diabetes in Phase 1 studies. In patients with type 2 diabetes given 100 mg to 300 mg once daily over a 16-day dosing period, reductions in RTG and increases in urinary glucose excretion were observed over the dosing period. In this study, plasma glucose declined in a dose-dependent fashion within the first day of dosing. In single-dose studies in healthy and type 2 diabetic subjects, treatment with canagliflozin 300 mg before a mixed-meal delayed intestinal glucose absorption and reduced postprandial glucose.
Cardiac Electrophysiology In a randomized, double-blind, placebo-controlled, active-comparator, 4-way crossover study, 60 healthy subjects were administered a single oral dose of canagliflozin 300 mg, canagliflozin 1,200 mg (4 times the maximum recommended dose), moxifloxacin, and placebo. No meaningful changes in QTc interval were observed with either the recommended dose of 300 mg or the 1,200 mg dose.
Pharmacokinetics
The pharmacokinetics of canagliflozin is similar in healthy subjects and patients with type 2 diabetes. Following single-dose oral administration of 100 mg and 300 mg of canagliflozin, peak plasma concentrations (median Tmax) of canagliflozin occurs within 1 to 2 hours post-dose. Plasma Cmax and AUC of canagliflozin increased in a dose-proportional manner from 50 mg to 300 mg. The apparent terminal half-life (t1/2) was 10.6 hours and 13.1 hours for the 100 mg and 300 mg doses, respectively. Steady-state was reached after 4 to 5 days of once-daily dosing with canagliflozin 100 mg to 300 mg. Canagliflozin does not exhibit time-dependent pharmacokinetics and accumulated in plasma up to 36% following multiple doses of 100 mg and 300 mg.
Absorption
The mean absolute oral bioavailability of canagliflozin is approximately 65%. Co-administration of a high-fat meal with canagliflozin had no effect on the pharmacokinetics of canagliflozin; therefore, canagliflozin may be taken with or without food. However, based on the potential to reduce postprandial plasma glucose excursions due to delayed intestinal glucose absorption, it is recommended that canagliflozin be taken before the first meal of the day [see DOSAGE AND ADMINISTRATION (2.1)].
Distribution
The mean steady-state volume of distribution of canagliflozin following a single intravenous infusion in healthy subjects was 119 L, suggesting extensive tissue distribution. Canagliflozin is extensively bound to proteins in plasma (99%), mainly to albumin. Protein binding is independent of canagliflozin plasma concentrations. Plasma protein binding is not meaningfully altered in patients with renal or hepatic impairment.
Metabolism
O-glucuronidation is the major metabolic elimination pathway for canagliflozin, which is mainly glucuronidated by UGT1A9 and UGT2B4 to two inactive O-glucuronide metabolites.
CYP3A4-mediated (oxidative) metabolism of canagliflozin is minimal (approximately 7%) in humans.
Excretion
Following administration of a single oral [14C] canagliflozin dose to healthy subjects, 41.5%, 7.0%, and 3.2% of the administered radioactive dose was recovered in feces as canagliflozin, a hydroxylated metabolite, and an O-glucuronide metabolite, respectively. Enterohepatic circulation of canagliflozin was negligible.
Approximately 33% of the administered radioactive dose was excreted in urine, mainly as O-glucuronide metabolites (30.5%). Less than 1% of the dose was excreted as unchanged canagliflozin in urine. Renal clearance of canagliflozin 100 mg and 300 mg doses ranged from 1.30 to 1.55 mL/min.
Mean systemic clearance of canagliflozin was approximately 192 mL/min in healthy subjects following intravenous administration.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Carcinogenicity was evaluated in 2-year studies conducted in CD1 mice and Sprague-Dawley rats.
- Canagliflozin did not increase the incidence of tumors in mice dosed at 10, 30, or 100 mg/kg (less than or equal to 14 times exposure from a 300 mg clinical dose).
- Testicular Leydig cell tumors, considered secondary to increased luteinizing hormone (LH), increased significantly in male rats at all doses tested (10, 30, and 100 mg/kg).
- In a 12-week clinical study, LH did not increase in males treated with canagliflozin.
- Renal tubular adenoma and carcinoma increased significantly in male and female rats dosed 100 mg/kg, or approximately 12-times exposure from a 300 mg clinical dose. Also, adrenal pheochromocytoma increased significantly in males and numerically in females dosed 100 mg/kg.
- Carbohydrate malabsorption associated with high doses of canagliflozin was considered a necessary proximal event in the emergence of renal and adrenal tumors in rats.
- Clinical studies have not demonstrated carbohydrate malabsorption in humans at canagliflozin doses of up to 2-times the recommended clinical dose of 300 mg.
Mutagenesis
- Canagliflozin was not mutagenic with or without metabolic activation in the Ames assay. Canagliflozin was mutagenic in the in vitro mouse lymphoma assay with but not without metabolic activation.
- Canagliflozin was not mutagenic or clastogenic in an in vivo oral micronucleus assay in rats and an in vivo oral Comet assay in rats.
Impairment of Fertility
- Canagliflozin had no effects on the ability of rats to mate and sire or maintain a litter up to the high dose of 100 mg/kg (approximately 14 times and 18 times the 300 mg clinical dose in males and females, respectively), although there were minor alterations in a number of reproductive parameters (decreased sperm velocity, increased number of abnormal sperm, slightly fewer corpora lutea, fewer implantation sites, and smaller litter sizes) at the highest dosage administered.
Animal Toxicology and/or Pharmacology
- In a juvenile toxicity study in which canagliflozin was dosed directly to young rats from postnatal day (PND) 21 until PND 90 at doses of 4, 20, 65, or 100 mg/kg, increased kidney weights and a dose-related increase in the incidence and severity renal pelvic and renal tubular dilatation were reported at all dose levels.
- Exposure at the lowest dose tested was greater than or equal to 0.5 times the maximum clinical dose of 300 mg. The renal pelvic dilatations observed in juvenile animals did not fully reverse within the 1-month recovery period. *Similar effects on the developing kidney were not seen when canagliflozin was administered to pregnant rats or rabbits during the period of organogenesis or during a study in which maternal rats were dosed from gestation day (GD) 6 through PND 21 and pups were indirectly exposed in utero and throughout lactation.
- In embryo-fetal development studies in rats and rabbits, canagliflozin was administered for intervals coinciding with the first trimester period of non-renal organogenesis in humans.
- No developmental toxicities were observed at any dose tested other than a slight increase in the number of fetuses with reduced ossification at a dose that was associated with maternal toxicity and that is approximately 19 times the human exposure to canagliflozin at the 300 mg clinical dose.
Clinical Studies
There is limited information regarding Canagliflozin Clinical Studies in the drug label.
How Supplied
100 mg tablets
- Are yellow, capsule-shaped, film-coated tablets with "CFZ" on one side and "100" on the other side.

300 mg tablets
- Are white, capsule-shaped, film-coated tablets with "CFZ" on one side and "300" on the other side.

Storage
- Store at 25°C (77°F); excursions permitted to 15 to 30°C (59 to 86°F).
Images
Drug Images
{{#ask: Page Name::Canagliflozin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Canagliflozin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Instructions
- Instruct patients to read the Medication Guide before starting canagliflozin therapy and to reread it each time the prescription is renewed.
- Inform patients of the potential risks and benefits of canagliflozin and of alternative modes of therapy. Also inform patients about the importance of adherence to dietary instructions, regular physical activity, periodic blood glucose monitoring and HbA1C testing, recognition and management of hypoglycemia and hyperglycemia, and assessment for diabetes complications. *Advise patients to seek medical advice promptly during periods of stress such as fever, trauma, infection, or surgery, as medication requirements may change.
- Instruct patients to take canagliflozin only as prescribed.
- If a dose is missed, advise patients to take it as soon as it is remembered unless it is almost time for the next dose, in which case patients should skip the missed dose and take the medicine at the next regularly scheduled time. Advise patients not to take two doses of canagliflozin at the same time.
- Inform patients that the most common adverse reactions associated with canagliflozin are genital mycotic infection, urinary tract infection, and increased urination.
- Inform female patients of child bearing age that the use of canagliflozin during pregnancy has not been studied in humans, and that canagliflozin should only be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Instruct patients to report pregnancies to their physicians as soon as possible.
- Inform nursing mothers to discontinue canagliflozin or nursing, taking into account the importance of drug to the mother.
Laboratory Tests
- Due to its mechanism of action, patients taking canagliflozin will test positive for glucose in their urine.
Hypotension
- Inform patients that symptomatic hypotension may occur with canagliflozin and advise them to contact their doctor if they experience such symptoms. *Inform patients that dehydration may increase the risk for hypotension, and to have adequate fluid intake.
Genital Mycotic Infections in Females (e.g., Vulvovaginitis)
- Inform female patients that vaginal yeast infection may occur and provide them with information on the signs and symptoms of vaginal yeast infection.
- Advise them of treatment options and when to seek medical advice.
Genital Mycotic Infections in Males (e.g., Balanitis or Balanoposthitis)
- Inform male patients that yeast infection of penis (e.g., balanitis or balanoposthitis) may occur, especially in uncircumcised males and patients with prior history.
- Provide them with information on the signs and symptoms of balanitis and balanoposthitis (rash or redness of the glans or foreskin of the penis). *Advise them of treatment options and when to seek medical advice.
Hypersensitivity Reactions
- Inform patients that serious hypersensitivity reactions such as urticaria and rash have been reported with canagliflozin.
- Advise patients to report immediately any signs or symptoms suggesting allergic reaction or angioedema, and to take no more drug until they have consulted prescribing physicians.
Urinary Tract Infections
- Inform patients of the potential for urinary tract infections.
- Provide them with information on the symptoms of urinary tract infections. Advise them to seek medical advice if such symptoms occur.
Precautions with Alcohol
Alcohol-Canagliflozin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Invokana
Look-Alike Drug Names
There is limited information regarding Canagliflozin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Canagliflozin |Label Name=Canagliflozin package.png
}}
{{#subobject:
|Label Page=Canagliflozin |Label Name=Canagliflozin package 2.png
}}
- Pages using duplicate arguments in template calls
- Pages with script errors
- Articles with changed ChemSpider identifier
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields