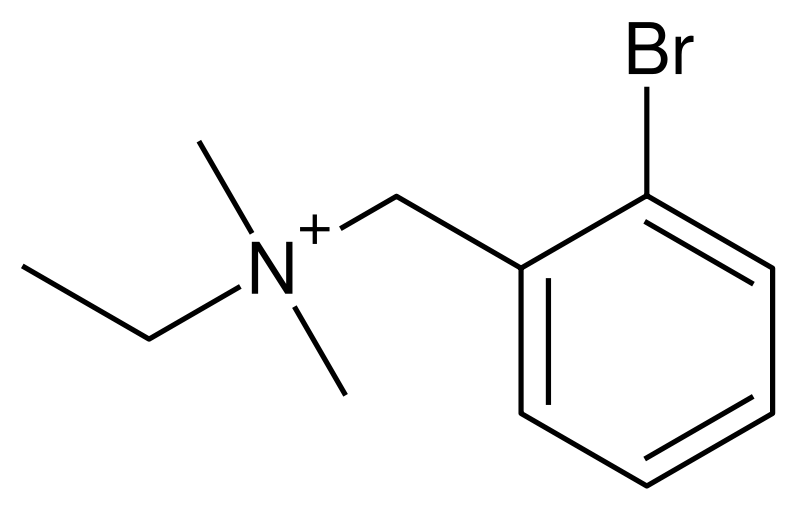
Bretylium
 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | IV, IM |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | NA |
| Protein binding | NA |
| Metabolism | None |
| Elimination half-life | 7-8 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C11H17BrN+ |
| Molar mass | 243.163 g/mol |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Bretylium (also bretylium tosylate) blocks the release of noradrenaline from the peripheral sympathetic nervous system, and is used in emergency medicine, cardiology, & other specialties for the acute management of ventricular tachycardia & ventricular fibrillation. It is contraindicated in patients with AV (atrioventricular) heart block or digoxin toxicity.
Bretylium is only used in an ICU setting and should not be used elsewhere due to its dramatic actions and its predominant side effect of hypotension.