Branched-chain amino acids
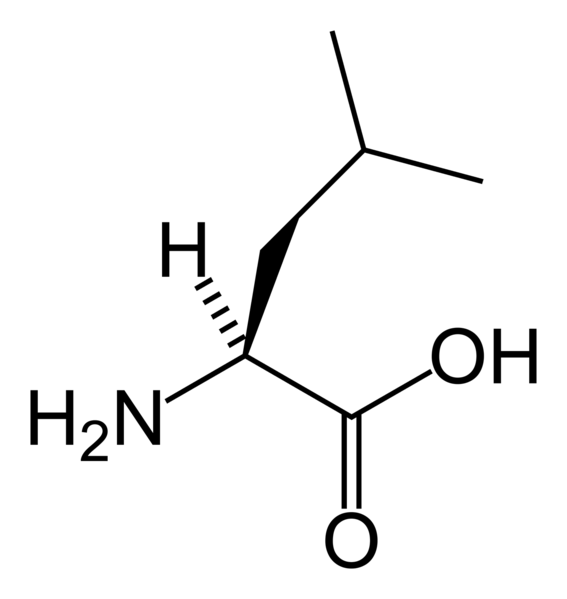


The phrase branched-chain amino acids or BCAA is sometimes used to refer to the amino acids having aliphatic side-chains that are non-linear. These are leucine, isoleucine and valine. The combination of these three essential amino acids make up approximately 1/3 of skeletal muscle in the human body, and play an important role in protein synthesis. BCAA’s are currently used clinically to aid in the recovery of burn victims, as well as for supplementation for strength athletes.
Metabolic Pathway
Description of Pathway
The BCAAs affect this metabolic pathway in three different manners. The first manner through which the metabolic pathway is affected is the increased production of insulin. It has been demonstrated that BCAA supplementation in accordance with carbohydrate intake following resistance exercise increases insulin output by 221%, which is much greater than the 66% supplementation without leucine. Leucine is the most readily oxidized BCAA and therefore the most effective at causing insulin secretion from the pancreas, and stimulating the metabolic pathway. This is important because insulin is the initiating factor that begins the signalling cascade. The second effect is outlined with the red arrows clearly indicating a phosphorylation cascade beginning with the activation of the Ras Rhed and ending with rpS6. The importance of rpS6 is that it induces mRNA translation. This translation leads to the production of proteins to build muscle. The other side of the pathway indicated by the blue arrow induces the action of eIF4G. This initiation factor causes the binding of mRNA within the ribosome thus providing the intial products needed for translation. This leads down to the final product via protein binding and phosphorylation, resulting in eIF4E which induces mRNA translation which in turn leads to chain elongation, and termination, resulting in net protein growth.
Importance of BCAAs
BCAAs play an important role in protein synthesis. In general terms, after a bout of resistance training the muscle will be in a catabolic state, with a protein synthesis deficit. This is because post exercise the MAPK signaling pathway is activated to induce muscle growth. While this is a pathway that will increase protein synthesis, it is not as effective as when combined with the BCAA signaling cascade. The two pathways act independent of each other. Because of this, when adequate amounts of BCAAs are ingested post workout (studies demonstrate that as little as 3g can be sufficient) the body is placed in a greater state of hypertrophy with a positive amount of protein synthesis. This is extremely important for athletes because it will decrease recovery time as it increases the rate at which lean body mass is gained. The use of BCAAs in burn victims results in the same positive protein synthesis. This allows victims to heal faster and return to normal activities at an increased rate, which is the treatment goal.
References
- Karlsson HK, Nilsson PA, Nilsson J, Chibalin AV, Zierath JR, Blomstrand E (2004). "Branched-chain amino acids increase p70S6k phosphorylation in human skeletal muscle after resistance exercise". Am. J. Physiol. Endocrinol. Metab. 287 (1): E1–7. doi:10.1152/ajpendo.00430.2003. PMID 14998784.
- Blomstrand E, Eliasson J, Karlsson HK, Köhnke R (2006). "Branched-chain amino acids activate key enzymes in protein synthesis after physical exercise". J. Nutr. 136 (1 Suppl): 269S–73S. PMID 16365096.
- Norton LE, Layman DK (2006). "Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise". J. Nutr. 136 (2): 533S–537S. PMID 16424142.
- "Testosterone Nation - BCAA and Athletic Performance". Retrieved 2007-05-25.
See also
External links
- Branched-chain+amino+acids at the US National Library of Medicine Medical Subject Headings (MeSH)
