Bivalirudin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alejandro Lemor, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Bivalirudin is a direct thrombin inhibitor that is FDA approved for the {{{indicationType}}} of anticoagulation in patients undergoing percutaneous transluminal coronary angioplasty (PTCA) and in patients with unstable angina or risk of heparin induced thrombocytopenia (HIT) undergoing percutaneous coronary intervention (PCI).. Common adverse reactions include bleeding, headache, thrombocytopenia and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Percutaneous Transluminal Coronary Angioplasty (PTCA)
- Dosing Information
- Initial dose: 0.75 mg/kg IV bolus
- Maintenance dose: 1.75 mg/kg/hr IV infusion for the duration of the procedure
- May be continued 4 hours post-procedure
- Five min after the bolus dose has been administered, an activated clotting time (ACT) should be performed and an additional bolus of 0.3 mg/kg should be given if needed.
Percutaneous Coronary Intervention (PCI)
- Dosing Information
- Initial dose: 0.75 mg/kg IV bolus
- Maintenance dose: 1.75 mg/kg/hr IV infusion for the duration of the procedure
- May be continued 4 hours post-procedure
- Five min after the bolus dose has been administered, an activated clotting time (ACT) should be performed and an additional bolus of 0.3 mg/kg should be given if needed.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Bivalirudin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Bivalirudin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Bivalirudin in pediatric patients.
Contraindications
- Active major bleeding;
- Hypersensitivity (e.g., anaphylaxis) to bivalirudin or its components.
Warnings
Bleeding Events
- Although most bleeding associated with the use of bivalirudin in PCI/PTCA occurs at the site of arterial puncture, hemorrhage can occur at any site.
- An unexplained fall in blood pressure or hematocrit should lead to serious consideration of a hemorrhagic event and cessation of bivalirudin administration.
- Bivalirudin should be used with caution in patients with disease states associated with an increased risk of bleeding.
Coronary Artery Brachytherapy
- An increased risk of thrombus formation, including fatal outcomes, has been associated with the use of bivalirudin in gamma brachytherapy.
- If a decision is made to use bivalirudin during brachytherapy procedures, maintain meticulous catheter technique, with frequent aspiration and flushing, paying special attention to minimizing conditions of stasis within the catheter or vessels.
Adverse Reactions
Clinical Trials Experience
Bleeding
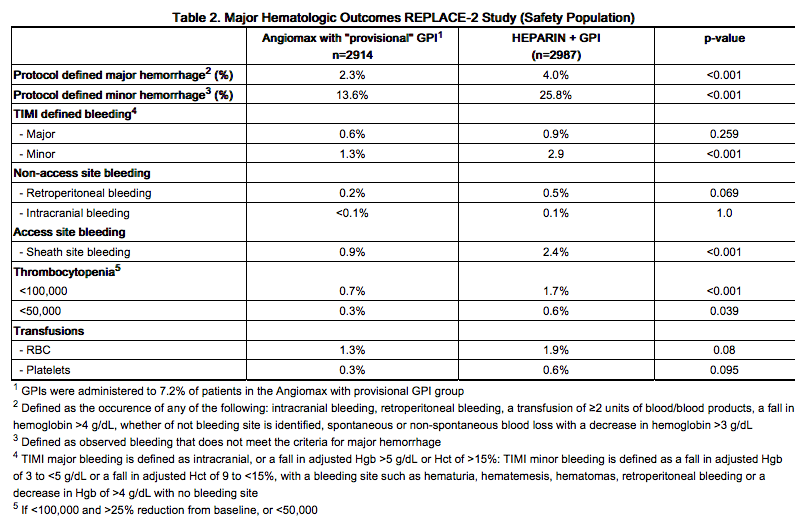
In 6010 patients undergoing PCI treated in the REPLACE-2 trial, bivalirudin patients exhibited statistically significantly lower rates of bleeding, transfusions, and thrombocytopenia as noted in Table 2.
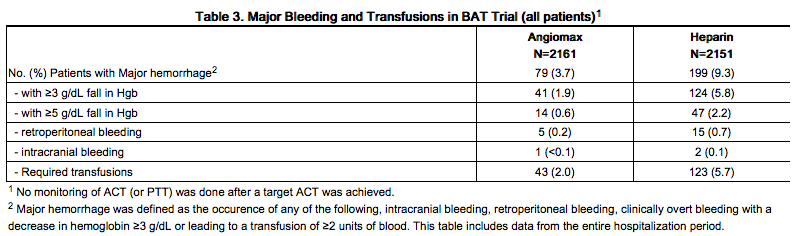
In 4312 patients undergoing PTCA for treatment of unstable angina in 2 randomized, double-blind studies comparing bivalirudin to heparin, bivalirudin patients exhibited lower rates of major bleeding and lower requirements for blood transfusions. The incidence of major bleeding is presented in Table 3. The incidence of major bleeding was lower in the bivalirudin group than in the heparin group.
In the AT-BAT study, of the 51 patients with HIT/HITTS, 1 patient who did not undergo PCI had major bleeding during CABG on the day following angiography. Nine patients had minor bleeding (mostly due to access site bleeding), and 2 patients developed thrombocytopenia.
Other Adverse Reactions
Adverse reactions, other than bleeding, observed in clinical trials were similar between the bivalirudin treated patients and the control groups. Adverse reactions (related adverse events ) seen in clinical studies in patients undergoing PCI and PTCA are shown in Tables 4 and 5.
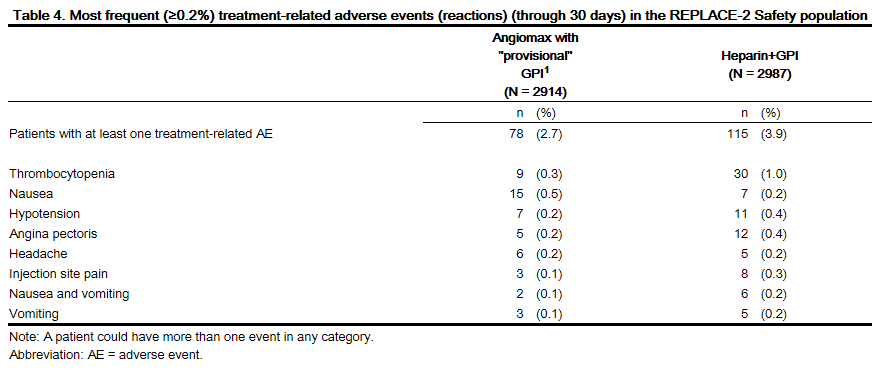
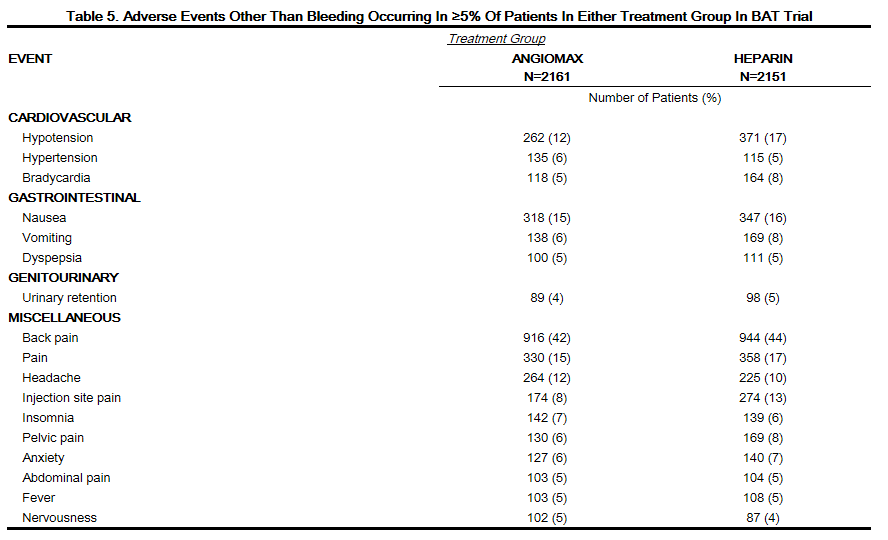
Serious, non-bleeding adverse events were experienced in 2% of 2161 bivalirudin-treated patients and 2% of 2151 heparin-treated patients. The following individual serious non-bleeding adverse events were rare (>0.1% to <1%) and similar in incidence between bivalirudin- and heparin-treated patients.
Body as a Whole
Cardiovascular
- Hypotension
- Syncope
- Vascular anomaly
- Ventricular fibrillation
Nervous
Respiratory
Urogenital
Immunogenicity/Re-Exposure
In in vitro studies, bivalirudin exhibited no platelet aggregation response against sera from patients with a history of HIT/HITTS. Among 494 subjects who received Angiomax in clinical trials and were tested for antibodies, 2 subjects had treatment-emergent positive bivalirudin antibody tests. Neither subject demonstrated clinical evidence of allergic or anaphylactic reactions and repeat testing was not performed. Nine additional patients who had initial positive tests were negative on repeat testing.
Postmarketing Experience
Because postmarketing adverse reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following adverse reactions have been identified during postapproval use of bivalirudin: fatal bleeding; hypersensitivity and allergic reactions including reports of anaphylaxis; lack of anticoagulant effect; thrombus formation during PCI with and without intracoronary brachytherapy, including reports of fatal outcomes.
Drug Interactions
- In clinical trials in patients undergoing PCI/PTCA, co-administration of bivalirudin with heparin, warfarin, thrombolytics, or GPIs was associated with increased risks of major bleeding events compared to patients not receiving these concomitant medications.
- There is no experience with co-administration of bivalirudin and plasma expanders such as dextran.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B Reproductive studies have been performed in rats at subcutaneous doses up to 150 mg/kg/day, (1.6 times the maximum recommended human dose based on body surface area) and rabbits at subcutaneous doses up to 150 mg/kg/day (3.2 times the maximum recommended human dose based on body surface area). These studies revealed no evidence of impaired fertility or harm to the fetus attributable to bivalirudin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
bivalirudin is intended for use with aspirin. Because of possible adverse effects on the neonate and the potential for increased maternal bleeding, particularly during the third trimester, bivalirudin and aspirin should be used together during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Bivalirudin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Bivalirudin during labor and delivery.
Nursing Mothers
It is not known whether bivalirudin is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when bivalirudin is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of bivalirudin in pediatric patients have not been established.
Geriatic Use
In studies of patients undergoing PCI, 44% were ≥65 years of age and 12% of patients were ≥75 years old. Elderly patients experienced more bleeding events than younger patients. Patients treated with bivalirudin experienced fewer bleeding events in each age stratum, compared to heparin.
Gender
There is no FDA guidance on the use of Bivalirudin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Bivalirudin with respect to specific racial populations.
Renal Impairment
The disposition of bivalirudin was studied in PTCA patients with mild, moderate and severe renal impairment. The clearance of bivalirudin was reduced approximately 20% in patients with moderate and severe renal impairment and was reduced approximately 80% in dialysis-dependent patients. The infusion dose of bivalirudin may need to be reduced, and anticoagulant status monitored in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Bivalirudin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Bivalirudin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Bivalirudin in patients who are immunocompromised.
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
Solution
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Y-Site
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Admixture
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Syringe
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
TPN/TNA
Compatible
- Solution 1
- Solution 2
- Solution 3
Not Tested
- Solution 1
- Solution 2
- Solution 3
Variable
- Solution 1
- Solution 2
- Solution 3
Incompatible
- Solution 1
- Solution 2
- Solution 3
Overdosage
- Cases of overdose of up to 10 times the recommended bolus or continuous infusion dose of bivalirudin have been reported in clinical trials and in postmarketing reports.
- A number of the reported overdoses were due to failure to adjust the infusion dose of bivalirudin in persons with renal dysfunction including persons on hemodialysis.
- Bleeding, as well as deaths due to hemorrhage, have been observed in some reports of overdose.
- In cases of suspected overdosage, discontinue bivalirudin immediately and monitor the patient closely for signs of bleeding. There is no antidote to bivalirudin. bivalirudin is hemodialyzable.
Pharmacology
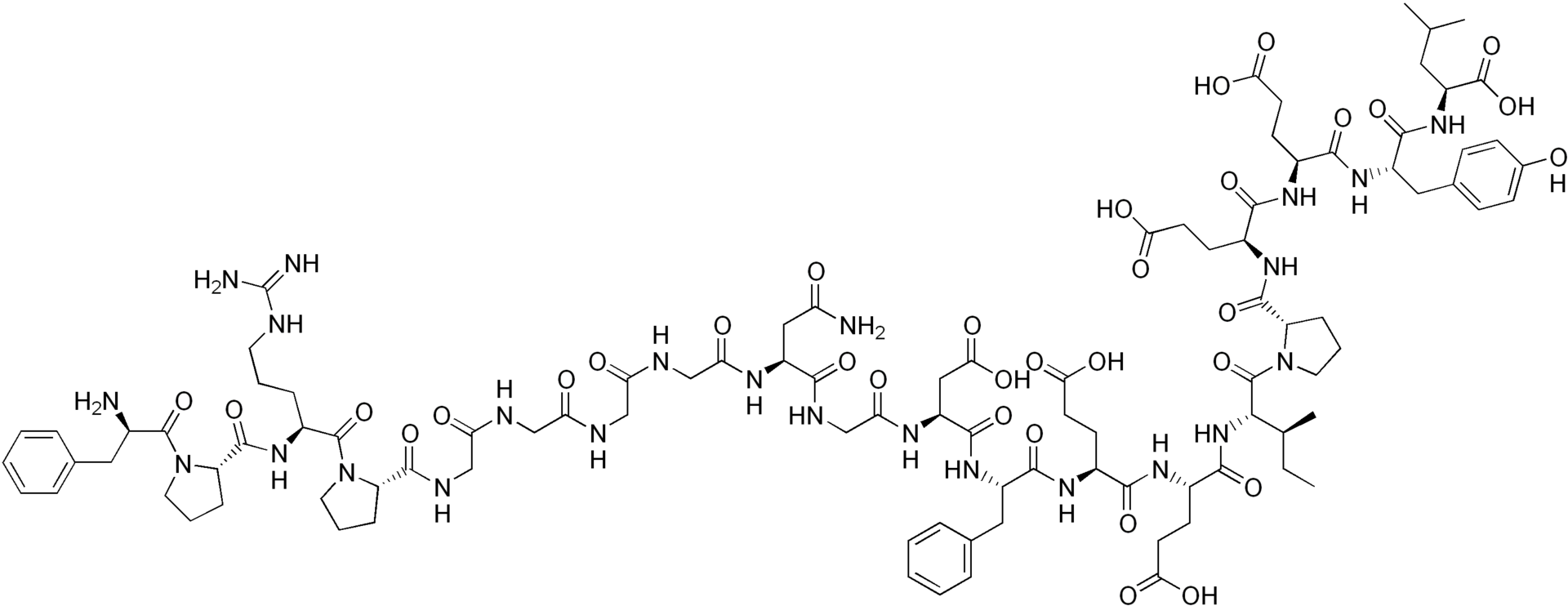
| |
Bivalirudin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | ? |
| ATC code | ? |
| PubChem | ? |
| Chemical data | |
| Formula | ? |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
(Description)
Structure
(Description with picture)
Pharmacodynamics
(Description)
Pharmacokinetics
(Description)
Nonclinical Toxicology
(Description)
Clinical Studies
Condition 1
(Description)
Condition 2
(Description)
Condition 3
(Description)
How Supplied
(Description)
Storage
There is limited information regarding Bivalirudin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Bivalirudin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Bivalirudin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
(Patient Counseling Information)
Precautions with Alcohol
Alcohol-Bivalirudin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Bivalirudin Brand Names in the drug label.
Look-Alike Drug Names
- (Paired Confused Name 1a) — (Paired Confused Name 1b)
- (Paired Confused Name 2a) — (Paired Confused Name 2b)
- (Paired Confused Name 3a) — (Paired Confused Name 3b)
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.