Artificial heart

|
WikiDoc Resources for Artificial heart |
|
Articles |
|---|
|
Most recent articles on Artificial heart Most cited articles on Artificial heart |
|
Media |
|
Powerpoint slides on Artificial heart |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Artificial heart at Clinical Trials.gov Trial results on Artificial heart Clinical Trials on Artificial heart at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Artificial heart NICE Guidance on Artificial heart
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Artificial heart Discussion groups on Artificial heart Patient Handouts on Artificial heart Directions to Hospitals Treating Artificial heart Risk calculators and risk factors for Artificial heart
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Artificial heart |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editors-In-Chief: Marvin J. Slepian, M.D.; University of Arizona [1]
Overview
An artificial heart is a prosthetic device that is implanted into the body to replace the biological heart. It is distinct from a cardiopulmonary bypass machine (CPB), which is an external device used to provide the functions of both the heart and the lungs. The CPB oxygenates the blood, and therefore does not need to be connected to both blood circuits. Also, a CPB is suitable only for a few hours use, while artificial hearts have been used for periods longer than a year (as of 2007).
Types
Total Artificial Heart (TAH) implantation involves the removal of the native heart. It is a surgical procedure similar to heart transplantation with a human donor heart.
Heart assist devices[1], also called cardiac assist devices or ventricular assist devices, differ, in that the patient’s heart is not removed during implantation. A heart assist device is a type of assisted circulation[2]. Assist devices may include either a Left Ventricular Assist Device (LVAD) or a Right Ventricular Assist Device (RVAD) or both. As opposed to TAH implantation, the assist device serves to provide only a part of the total cardiac output of the patient’s heart.
Origins
A synthetic replacement for the heart remains one of the long-sought holy grails of modern medicine. The obvious benefit of a functional artificial heart would be to lower the need for heart transplants, because the demand for donor hearts (as it is for all organs) always greatly exceeds supply.
Although the heart is conceptually simple (basically a muscle that functions as a pump), it embodies subtleties that defy straightforward emulation with synthetic materials and power supplies. Consequences of these issues include severe foreign-body rejection and external batteries that limit patient mobility. These complications limited the lifespan of early human recipients to hours or days.
Early designs
A heart-lung machine was used in 1953 during the first successful open heart surgery. Dr. John Heysham Gibbon performed the operation and developed the heart-lung substitute himself. [3] Whether this device could be considered as an artificial heart is a subject of debate.
The scientific interest for the development of a solution for heart disease developed in different research groups worldwide.
Early designs of total artificial hearts
In Russia in 1937 V.P. Demichov implanted TAH in dogs. Motor roller types TAH (inside the chest) with the driver shaft of which carried through the sternum.
In 1957 Tet Akutsu and Willem Kolff initiated their extended TAH research at the Cleveland Clinic.
In 1958 Domingo Liotta started the studies of TAH replacement at Lyon, France and in 1959-60 at the National University of Cordoba, Argentina. He presented his work at the meeting of the American Society for Artificial Internal Organs meeting held in Atlantic City in March 1961. On that meeting Dr Liotta described the implantation of three types of orthotopic (inside the pericardial sac) TAH in dogs, each of which used a different source of external energy: an implantable electric motor, an implantable rotating pump with an external electric motor and a pneumatic pump.[4] [5]
Early clinical application of assisted circulation and total artificial heart
The Left Ventricular Assist Device (LVAD) system was created by Domingo Liotta at Baylor University College of Medicine in Houston in 1962. [6]
First clinical application of an intrathoracic pump
In the evening of July 19 1963 E. Stanley Crawford and Domingo Liotta implanted the first clinical LVAD at the Methodist Hospital in Houston, Texas in a patient who had a cardiac arrest after surgery. The patient survived for 4 days under mechanical support but didn't recover from the complications of the cardiac arrest, finally the pump was discontinued and the patient died.
First clinical application of a paracorporeal pump

In the afternoon of April 21 1966 Michael DeBakey and Domingo Liotta implanted the first clinical LVAD in a paracorporeal position (the external pump rests at the side of the patient) at the Methodist Hospital in Houston in a patient with cardiogenic shock after heart surgery. The patient developed neurological and pulmonary complications and died after few days of LVAD mechanical support.
In October 1966 Michael E. DeBakey and Domingo Liotta implanted the paracorporeal Liotta-DeBakey LVAD in a new patient who recovered well, and was discharged from hospital after 10 days of mechanical support. thus constituting the first successful use of an LVAD for postcardiotomy shock.
First clinical implantation of a total artificial heart
In the afternoon of April 4 1969 Denton A. Cooley and Domingo Liotta replaced a dying man’s heart with a mechanical heart inside the chest at the Texas Heart Institute in Houston as a bridge for a transplant. The patient woke up and recovered well. After 64 hours the pneumatic powered artificial heart was removed and replaced by a donor heart. Replacing the artificial heart proved to be a bad decision, however; thirty-two hours after transplantation the patient died of what was later proved to be an acute pulmonary infection, extended to both lungs, caused by fungi, most likely caused by an immunosuppressive drugs complication. If they left the artificial heart in place the patient might not have died. [7]
The original prototype of Liotta-Cooley artificial heart used in this historic operation is prominently displayed in The Smithsonian Museum Treasures of American Historyin Washington,DC.
The first patented artificial heart was invented by Paul Winchell in 1963 [2]. Winchell subsequently assigned the patent to the University of Utah, where Robert Jarvik ultimately used it as the model for his Jarvik-7. Jarvik's designs improved the device, but his patients succumbed after brief trials. His first Jarvik-7 patient, 61-year-old retired dentist Barney Clark, survived for 112 days after it was implanted at the University of Utah on December 2, 1982. One of the innovations of the Jarvik-7 was the inner coating of rough material, developed by David Gernes. This coating helped the blood to clot and coat the inside of the device, enabling a more natural blood flow.
-
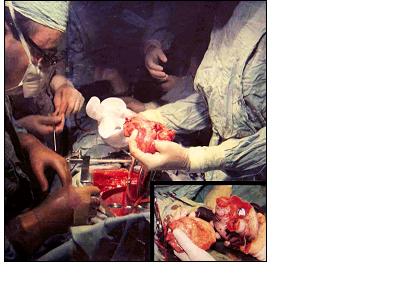
Historical Operation, the first in medical history. Total heart Replacement with an Artificial Heart (orthotopic position, inside the pericardial sac). On the left, Dr. Liotta; in the center of the picture, the empty pericardial sac of the patient, Mr. H. Karp. On the right, the hands of Dr. Cooley holding Mr. Karp’s heart and the artificial heart just before implantation. Texas Heart Institute, Houston (April 4 1969)Corner picture: Dr. Cooley is holding both the removed artificial heart and the donor heart. (April 7, 1969).
-
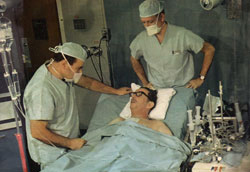
Dr. Liotta is talking to Mr. Karp and Dr. Cooley is observing (April 5 1969)
-
The CardioWest™ temporary Total Artificial Heart
-
An artificial heart displayed at the London science museum]]
The development of permanent, implantable, electrically powered artificial hearts
In the mid 1980s, artificial hearts were powered by dishwasher-sized pneumatic power sources whose lineage went back to Alpha-Laval milking machines. Moreover, two sizeable catheters had to cross the body wall to carry the pneumatic pulses to the implanted heart, greatly increasing the risk of infection. To speed development of a new generation of technologies, the National Heart, Lung, and Blood Institute opened a competition for implantable electrically powered artificial hearts. Three groups received funding: Cleveland Clinic in Cleveland, Ohio; the College of Medicine of Pennsylvania State University (Penn State Hershey Medical Center) in Hershey, Pennsylvania; and Abiomed, Inc. of Danvers, Massachusetts. Despite considerable progress, the Cleveland program was discontinued after the first five years.
The Penn State Heart is of an electromechanical design with a brushless DC motor positioned between polyurethane, sac-style right and left ventricles. As a roller screw translates rotary motion to linear motion, pusher plates alternately activate the right and left ventricles. Four Bjork-Shiley tilting disc valves ensure unidirectional blood flow. A digital electronic control system that indirectly senses arterial and left atrial pressure provides an adequate blood flow while balancing the output of the two ventricles. Energy from nickel-cadmium battery carried in a shoulder bag or on a belt is transfered to the implanted motor wirelessly, through inductive coupling. This artificial heart has been extensively tested on artificial circulatory systems and implanted in over 50 calves, with animal survival of nearly one year.
Abiomed's AbioCor is the first fully implantable total artificial heart, which unlike previous total artificial hearts (such as the Jarvik 7) does not require wires to external devices.[8] This replacement heart uses an electrohydraulic design with a high-speed impeller pump that pressurizes a hydraulic fluid. An energized ported valve shuttles the fluid, alternately actuating diaphragm-style left and right ventricles. Polymeric trileaflet valves ensure unidirectional blood flow with a low pressure gradient and good longevity. State-of-the art transcutaneous energy transfer eliminates the need for electric wires crossing the chest wall.
Hiroaki Harasaki of the Cleveland Clinic developed two important improvements for the artificial heart and projected future artificial organs. The two patented inventions solved major obstacles for any fully implanted artificial organs and materials. The first was a non-clotting surface material which significantly reduces the risk of rejection of the organ by the patient's immune system. The second development, which required the collaboration of many disciplines, was an implantable power source which does not create tissue-damaging heat.
The first AbioCor to be surgically implanted in a patient was on July 3, 2001.[9] The AbioCor is made of titanium and plastic with a weight of 2 pounds and its internal battery can be recharged with a transduction device that sends power through the skin.[9] The internal battery lasts for half an hour and a wearable external battery pack lasts for 4 hours.[10]
The FDA announced on September 5, 2006 that the AbioCor could be implanted for humanitarian uses after the device had been tested on 15 patients.[11] It is intended for critically ill patients who can not receive a heart transplant.[11] Some limitations of the current AbioCor are that its size makes it suitable for only about 50% of the male population and its useful life is only 1 to 2 years.[12] By combining its valved ventricles with the control technology and roller screw developed at Penn State, Abiomed has designed a smaller, more stable heart, the AbioCor II. This pump, which should be implantable in most men and 50% of women with a life span of up to 5 years,[12] had animal trials in 2005, and the company hopes to get FDA approval for human use in 2008.[13]
After about 90 people received the Jarvik device, the implantation of artificial hearts was banned for permanent use in patients with heart failure, because most of the recipients could not live more than half a year. However, it is used temporarily for some heart transplantation candidates who cannot find a natural heart immediately but urgently need an efficiently working heart.
Recent developments
On July 2, 2001, Robert Tools received the AbioCor Implantable Replacement Heart produced by the AbioMed company of Danvers, Massachusetts. It was the first completely self-contained artificial heart transplant. The surgery was done by University of Louisville doctors at Jewish Hospital in Louisville, Kentucky. Tom Christerson survived for 17 months after another AbioCor transplant. On September 6, 2006 the AbioCor device became the first fully implantable artificial heart to be approved under 'Humanitarian Use Device' rules.[14]
The 'CardioWest' temporary Total Artificial Heart (TAH‑t) was developed from the Jarvik-7 by University of Arizona researchers and approved for use in 2004.[15] It is the first implantable artificial heart to be approved by the U.S. Food and Drug Administration, and has also been approved by the CE. The TAH-t is used only in patients with end stage biventricular failure as a way to improve life expectancy while they are waiting for a heart transplant. In a pivotal clinical study, these patients were successfully transplanted 79% of the time;[16], One-year and five-year survival rates after heart transplant among these patients were 86 and 64 percent. The longest TAH‑t implantation so far went 620 days (20.4 months).[17] There are several medical centers where this device can be implanted:
United States: [18]
- University Medical Center (Tucson, AZ) [3]
- Cleveland Clinic (Cleveland, OH) [4]
- Virginia Commonwealth University Health System (Richmond, VA) [5]
- Aurora St. Luke's (Milwaukee, WI) [6]
- University of Michigan Health System (Ann Arbor, MI) [7]
- Penn State Hershey Medical Center (Hershey, PA) [8]
- Ohio State University Medical Center (Columbus, OH) [9]
- Hospital of the University of Pennsylvania (Philadelphia, PA) [10]
- Barnes Jewish Hospital (St. Louis, MO) [11]
- University of Rochester Medical Center (Rochester, NY) [12]
Canada:
- Montreal Heart Institute (Quebec, Canada) [13]
Europe:
- Groupe Hospitalier La Pitié-Salpêtrière (Paris, France) [14]
- Hôpital Guillaume et René Laennec (Nantes, France) [15]
- Deutsches Herzzentrum Berlin / German Heart Institute Berlin (Berlin, Germany) [16]
- Herz-und Diabeteszentrum Nordrhein Westfalen / Heart and Diabetes Center (Bad Oeynhausen, Germany) [17]
- Herzzentrum Leipzig GmbH Universitaetsklinik (Leipzig, Germany) [18]
- Universitäts Klinikum Freiburg (Freiburg, Germany) [19]
- Universitätsklinikum Münster (Munster, Germany) [20]
- Herzzentrum Köln (Cologne, Germany) [21]
- University Hospital Munich (Munich, Germany) [22]
- Friedrich-Alexander University Hospital (Nuremberg, Germany) [23]
In August 2006, an artificial heart was implanted into a 15-year old girl at the Stollery Children's Hospital in Edmonton,Alberta, Canada. It was intended to act as a temporary fixture until a donor heart could be found. Instead, the artificial heart (called a Berlin Heart) allowed for natural processes to occur and her heart healed on its own. After 146 days the Berlin Heart was removed and the girl's heart was able to function properly on its own.[19].
With increased understanding of the heart and continuing improvements in prosthetics engineering, computer science, electronics, battery technology, and fuel cells, a practical artificial heart may be a reality in the 21st century.
FDA-Approved Artificial Hearts
CardioWest™ temporary Total Artificial Heart
The CardioWest™ temporary Total Artificial Heart (TAH-t) (SynCardia Systems, Inc.) is the world’s first and only FDA-approved Total Artificial Heart. It received FDA approval on Oct. 15, 2004, following a 10-year pivotal clinical study.[20]
Originally designed as a permanent replacement heart, it is currently approved as a bridge to human heart transplant for patients dying because both sides of their hearts are failing (irreversible end stage biventricular failure).[21] There have been more than 770 implants of the CardioWest artificial heart, accounting for more than 150 patient years of life on this device.[22]
In the 10-year pivotal clinical study of the CardioWest artificial heart 79% of patients receiving the artificial heart survived to transplant (New England Journal of Medicine 2004; 351: 859-867).[23] This is the highest bridge-to-transplant rate for any heart device in the world.[24] See FDA Summary of Safety and Effectiveness
Heart assist devices
Patients who have some remaining heart function but who can no longer live normally may be candidates for ventricular assist devices which do not replace the heart, but boost its output. The first heart assist device was FDA approved in 1994, and two more received approval in 1998.[25] While the original assist devices emulated the pulsating heart newer versions, such as the Heartmate II,[26] developed by the Texas Heart Institute of Houston, Texas, provide continuous flow. These pumps (which may be cetrifugal or axial flow) are smaller and potentially more durable and long-lasting than the current generation of total heart replacement pumps. Several continuous flow ventricular assist devices have been approved for use in the European Union and as at August 2007 were undergoing clinical trials for FDA approval.
References
- ↑ Anonymous (2024), Heart assist devices (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Anonymous (2024), Assisted circulation (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ George B. Griffenhagen and Calvin H. Hughes. The History of the Mechanical Heart. Smithsonian Report for 1955, (Pub. 4241): 339-356, 1956.
- ↑ Artificial Heart in the chest: Preliminary report. Trans.Amer. Soc. Inter. Organs, 1961,7:318
- ↑ Ablation experimentale et replacement du coeur par un coer artificial intra-thoracique. Lyon Cirurgical,1961, 57:704
- ↑ Prolonged Assisted circulation after cardiac or aortic surgery. Prolonged partial left ventricular bypass by means of intracorporeal circulation. This paper was finalist in: The Young Investigators Award Contest of the American College of Cardiology. Denver, May 1962 Am.J. Cardiol. 1963,12:399-404
- ↑ Orthotopic cardiac prosthesis for two-staged cardiac replacement. Am J Cardio 1969;24:723-30.
- ↑ "AbioCor latest innovation in crowded field". CNN.com. 2001-07-03. Retrieved 2008-07-13.
- ↑ 9.0 9.1 "Patient gets first totally implanted artificial heart". CNN.com. 2001-07-03. Retrieved 2008-07-13.
- ↑ "AbioCor FAQs". abiomed.com. Retrieved 2008-07-13.
- ↑ 11.0 11.1 "FDA Approves First Totally Implanted Permanent Artificial Heart for Humanitarian Uses". FDA.gov. 2006-09-05. Retrieved 2008-07-13.
- ↑ 12.0 12.1 "Will We Merge With Machines?". popsci.com. 2005-08-01. Retrieved 2008-07-13.
- ↑ "14th Artificial Heart Patient Dies: A Newsmaker Interview With Robert Kung, PhD". medscape.com. 2004-11-11. Retrieved 2008-07-13.
- ↑ FDA Approves First Totally Implanted Permanent Artificial Heart for Humanitarian Uses at FDA.gov
- ↑ Home Page at CardioWest
- ↑ Cardiac replacement with a total artificial heart as a bridge to transplantation at the National Institutes of Health
- ↑ Current status of the total artificial heart at Elsevier.com
- ↑ "CardioWest temporary Total Artificial Heart" (Press release). SynCardia Systems, Inc. 2007-01-18. Retrieved 2007-04-26.
- ↑ Capital Health: One year later: Berlin Heart bridges patient back to health
- ↑ http://www.fda.gov/cdrh/mda/docs/p030011.html
- ↑ http://www.fda.gov/cdrh/mda/docs/p030011.html
- ↑ http://www.syncardia.com
- ↑ http://content.nejm.org/cgi/content/short/351/9/859
- ↑ http://www.syncardia.com
- ↑ FDA APPROVES TWO PORTABLE HEART-ASSIST DEVICES at FDA.gov
- ↑ An Artificial Heart That Doesn't Beat at TechnologyReview.com
See Also
External links
- Artificial hearts and heart assist devices currently in use

![An artificial heart displayed at the London science museum]]](/images/2/21/Artificial-heart-london.JPG)