Artemether lumefantrine adverse reactions
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Adverse Reactions
Serious Adverse Reactions
The following serious and otherwise important adverse reactions are discussed in greater detail in other sections of labeling: Hypersensitivity Reactions [see Contraindications and Postmarketing Experience ].
Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rate observed in practice.
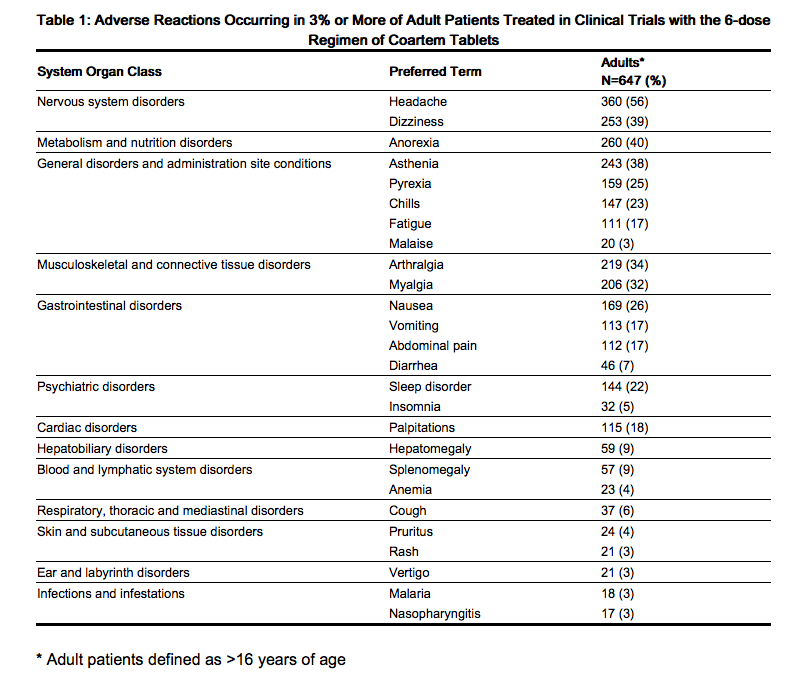
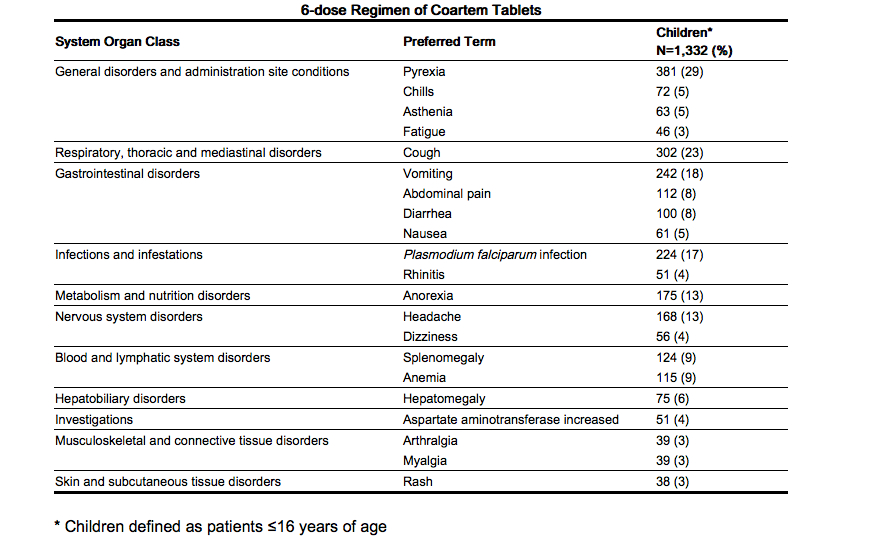
The data described below reflect exposure to a 6-dose regimen of Coartem Tablets in 1,979 patients including 647 adults (older than 16 years) and 1,332 children (16 years and younger). For the 6-dose regimen, Coartem Tablets was studied in active-controlled (366 patients) and non-controlled, open-label trials (1,613 patients). The 6-dose Coartem Tablets population was patients with malaria between ages 2 months and 71 years: 67% (1,332) were 16 years and younger and 33% (647) were older than 16 years. Males represented 73% and 53% of the adult and pediatric populations, respectively. The majority of adult patients were enrolled in studies in Thailand, while the majority of pediatric patients were enrolled in Africa.
Tables 1 and 2 show the most frequently reported adverse reactions (≥3%) in adults and children respectively who received the 6-dose regimen of Coartem Tablets. Adverse reactions collected in clinical trials included signs and symptoms at baseline but only treatment emergent adverse events, defined as events that appeared or worsened after the start of treatment, are presented below. In adults, the most frequently reported adverse reactions were headache, anorexia, dizziness, and asthenia. In children, the adverse reactions were pyrexia, cough, vomiting, anorexia, and headache. Most adverse reactions were mild, did not lead to discontinuation of study medication, and resolved.
In limited comparative studies, the adverse reaction profile of Coartem Tablets appeared similar to that of another antimalarial regimen.
Discontinuation of Coartem Tablets due to adverse drug reactions occurred in 1.1% of patients treated with the 6-dose regimen overall: 0.2% (1/647) in adults and 1.6% (21/1,332) in children.
 |
 |
Clinically significant adverse reactions reported in adults and/or children treated with the 6-dose regimen of Coartem Tablets which occurred in clinical studies at <3% regardless of causality are listed below:
Blood and lymphatic system disorders
Ear and labyrinth disorders
Eye disorders:
Gastrointestinal disorders
Constipation, dyspepsia, dysphagia, peptic ulcer]
General disorders
Gait disturbance
Infections and infestations
Abscess, acrodermatitis, bronchitis, ear infection, gastroenteritis, helminthic infection, hookworm infection, impetigo, influenza, lower respiratory tract infection, malaria,nasopharyngitis, oral herpes, pneumonia, respiratory tract infection, subcutaneous abscess, upper respiratory tract infection, urinary tract infection
Investigations
Alanine aminotransferase increased, aspartate aminotransferase increased, hematocrit decreased, lymphocyte morphology abnormal, platelet count decreased, platelet count increased, white blood cell count decreased, white blood cell count increased
Metabolism and nutrition disorders
Hypokalemia
Musculoskeletal and connective tissue disorders
Back pain
Nervous system disorders
Ataxia, clonus, fine motor delay, hyperreflexia, hypoaesthesia, nystagmus, tremor
Psychiatric disorders
Agitation, mood swings
Renal and urinary disorders
Hematuria, proteinuria
Respiratory, thoracic and mediastinal disorders
Asthma, pharyngo-laryngeal pain
Skin and subcutaneous tissue disorders
Urticaria[1]
References
- ↑ "COARTEM (ARTEMETHER AND LUMEFANTRINE) TABLET [NOVARTIS PHARMACEUTICALS CORPORATION]". Text " accessdate " ignored (help)
Adapted from the FDA Package Insert.