Aplastic anemia pathophysiology: Difference between revisions
Nazia Fuad (talk | contribs) No edit summary |
Iqra Qamar (talk | contribs) |
||
| (7 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
Please help WikiDoc by adding content here. It's easy! Click [[Help:How_to_Edit_a_Page|here]] to learn about editing. | Please help WikiDoc by adding content here. It's easy! Click [[Help:How_to_Edit_a_Page|here]] to learn about editing. | ||
==Overview== | ==Overview== | ||
[[Bone marrow]] is a spongy tissue, found within the spongy or cancellous portions of bones. It is higly vascularized and richly innervated [[Bone marrow]] is the primary site of [[hematopoiesis]] and is composed of [[Hematopoietic cell|hematopoietic cells]], marrow adipose tissue, and [[Stromal cell|stromal cells]]. The most defenitive feature in [[pathophysiology]] of aplastic anemia is loss of [[Hematopoietic stem cell|hematopoietic stem cells.]] It may be in the form of hematopoietic failure or immune mediated destruction of [[bone marrow]]. Drugs, [[chemicals]], [[viruses]], and different kind of [[mutations]] change the immunologic appearance of HSCs resulting in [[Autoimmune|autoimmune destruction]] of [[Bone Marrow cells|marrow cells]]. AA may develop gradually into other [[hematologic]] disorder which include [[paroxysmal nocturnal hemoglobinuria]] [PNH], [[Myelodysplastic syndrome|myelodysplastic syndromes]] [MDS] and [[Acute myeloid leukemia (generic term)|acute myeloid leukemia]] [AML]). Clonal evolution in AA can occur due to [[mutations]] or [[Cytogenetics|cytogenetic]] abnormalities. The [[Gene|genes]] that are commonly found to be mutated are ''DMNT3A,'' ''ASXL1,'' ''BCOR,'' ''BCORL1,'' ''PIGA''. | |||
==Pathophysiology== | ==Pathophysiology== | ||
=== Physiology === | === Physiology === | ||
The normal physiology of bone marrow can be understood as follows:<ref name="pmid2406826">{{cite journal |vauthors=Hays K |title=Physiology of normal bone marrow |journal=Semin Oncol Nurs |volume=6 |issue=1 |pages=3–8 |date=February 1990 |pmid=2406826 |doi= |url=}}</ref> | The normal physiology of [[bone marrow]] can be understood as follows:<ref name="pmid2406826">{{cite journal |vauthors=Hays K |title=Physiology of normal bone marrow |journal=Semin Oncol Nurs |volume=6 |issue=1 |pages=3–8 |date=February 1990 |pmid=2406826 |doi= |url=}}</ref> | ||
* | * Bone marrow is a spongy tissue, found within the spongy or [[Cancellous bone|cancellous portions of bones]] | ||
* It is higly vascularized and richly innervated | * It is higly vascularized and richly innervated | ||
* Bone marrow is the primary site of hematopoiesis | * [[Bone marrow]] is the primary site of [[hematopoiesis]]. | ||
* It is composed of hematopoietic cells, marrow adipose tissue, and stromal cells. | * It is composed of [[Hematopoietic cell|hematopoietic cells]], marrow [[adipose tissue]], and [[Stromal cells|stromal cells]]. | ||
* Hematopoietic stem cells (HSC) in the bone marrow are the source of all mature cells in the peripheral blood and tissues and are multipotent. | * [[Hematopoietic stem cells]] (HSC) in the [[bone marrow]] are the source of all mature cells in the peripheral blood and tissues and are [[Multipotent|multipotent]]. | ||
* HSC are recognized and isolated according to their immunophenotype. | * HSC are recognized and isolated according to their [[immunophenotype]]. | ||
* HSCs make a small population within the CD34+/CD38 fraction of bone marrow cells. | * HSCs make a small population within the CD34+/CD38 fraction of [[bone marrow cells]]. | ||
* The hematopoiesis is controlled by a various regulatory mechanisms, including growth factors. | * The [[hematopoiesis]] is controlled by a various regulatory mechanisms, including [[growth factors]]. | ||
* The normal bone marrow structure can be damaged or displaced by aplastic anemia, malignancies or infections. | * The normal [[bone marrow]] structure can be damaged or displaced by aplastic anemia, [[malignancies]] or [[infections]]. | ||
* This leads to decrease production of blood cells and blood platelets. | * This leads to decrease production of [[blood cells]] and blood [[platelets]]. | ||
. | . | ||
| Line 26: | Line 27: | ||
|[[File:Aplasticanemia.jpg |400px|thumb|right|Image yellow fat ladden marrow https://www.wikidoc.org/index.php/File:Aplasticanemia.jpg source:By Wmheric [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)] [Public domain], from Wikimedia Commons]]] | |[[File:Aplasticanemia.jpg |400px|thumb|right|Image yellow fat ladden marrow https://www.wikidoc.org/index.php/File:Aplasticanemia.jpg source:By Wmheric [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)] [Public domain], from Wikimedia Commons]]] | ||
|} | |} | ||
The most defenitive feature in pathophysiology of aplastic anemia is loss of hematopoietic stem cells.<ref name="pmid18024605">{{cite journal |vauthors=Bacigalupo A |title=Aplastic anemia: pathogenesis and treatment |journal=Hematology Am Soc Hematol Educ Program |volume= |issue= |pages=23–8 |date=2007 |pmid=18024605 |doi=10.1182/asheducation-2007.1.23 |url=}}</ref><ref name="Brodsky2000">{{cite journal|last1=Brodsky|first1=R. A.|title=Aplastic Anemia: Pathophysiology and Treatment|journal=Journal of the National Cancer Institute|volume=92|issue=9|year=2000|pages=754–754|issn=14602105|doi=10.1093/jnci/92.9.754}}</ref> | The most defenitive feature in pathophysiology of aplastic anemia is loss of [[hematopoietic stem cells]].<ref name="pmid18024605">{{cite journal |vauthors=Bacigalupo A |title=Aplastic anemia: pathogenesis and treatment |journal=Hematology Am Soc Hematol Educ Program |volume= |issue= |pages=23–8 |date=2007 |pmid=18024605 |doi=10.1182/asheducation-2007.1.23 |url=}}</ref><ref name="Brodsky2000">{{cite journal|last1=Brodsky|first1=R. A.|title=Aplastic Anemia: Pathophysiology and Treatment|journal=Journal of the National Cancer Institute|volume=92|issue=9|year=2000|pages=754–754|issn=14602105|doi=10.1093/jnci/92.9.754}}</ref> | ||
Pathophysiologic mechanisms that result in loss of HSCs and cause aplastic anemia include: | Pathophysiologic mechanisms that result in loss of HSCs and cause aplastic anemia include: | ||
| Line 32: | Line 33: | ||
==== Hematopoietic Failure ==== | ==== Hematopoietic Failure ==== | ||
* CD34 cells are almost absent aplastic anemia. | * CD34 cells are almost absent aplastic anemia. | ||
* Progenitor cells capable of forming erythroid, myeloid, and megakaryocytic are greatly reduced. | * [[Progenitor cells]] capable of forming [[erythroid]], [[Myeloid|myeloid,]] and [[Megakaryocyte|megakaryocytic]] are greatly reduced. | ||
* The primitive hematopoietic cells which are closely related to stem cells are consistently deficient. | * The primitive [[Hematopoietic cell|hematopoietic cells]] which are closely related to [[stem cells]] are consistently deficient. | ||
* The white blood cells in aplastic anemia have short telomeres. | * The white blood cells in aplastic anemia have short telomeres. | ||
* Telomeres are repeats at the end of eukaryotic chromosome and are essential for chromosome protection and complete DNA replication. | * Telomeres are repeats at the end of [[Eukaryotic chromosome structure|eukaryotic chromosome]] and are essential for [[chromosome]] protection and complete DNA replication. | ||
==== Immune-mediated T-cell destruction of marrow ==== | ==== Immune-mediated T-cell destruction of marrow ==== | ||
** In patients with acquired aplastic anemia, lymphocytes are responsible for the destruction of the hematopoietic cells. | ** [[Drugs|Drugs,]] [[chemicals]], [[viruses]], and different kind of [[mutations]] change the immunologic appearance of HSCs resulting in [[autoimmune]] destruction of [[Bone marrow|marrow cells.]]<ref name="Young2002" /> | ||
** These T cells produces an inhibitory factor, | ** In patients with acquired aplastic anemia, [[lymphocytes]] are responsible for the destruction of the [[Hematopoietic cell|hematopoietic cells]]. | ||
** CD4+CD25+FOXP3+ regulatory T cells are deficient in these patients, similar to what is seen in other autoimmune conditions. | ** These T cells produces an inhibitory factor, [[interferons]] , [[Tumour necrosis factor|tumor necrosis factor,]] and [[interleukin-2]], resulting in [[hematopoietic cell]] death by [[apoptosis]]. | ||
** Deficiency of these regulatory T cells result in increase of T-bet protein levels in T cells, increased interferon (IFN)-γ,2 and stem cell destruction. | ** CD4+CD25+FOXP3+ regulatory T cells are deficient in these patients, similar to what is seen in other [[autoimmune]] conditions. | ||
** Increased immune response, including tumor necrosis factor -α, IFNγ, and interleukin-6, are also very common in AA patients. | ** Deficiency of these regulatory T cells result in increase of T-bet protein levels in T cells, increased interferon (IFN)-γ,2 and [[stem cell]] destruction. | ||
** Increased immune response, including [[tumor necrosis factor]] -α, IFNγ, and [[interleukin-6]], are also very common in AA patients. | |||
==== Clonal Evolution ==== | ==== Clonal Evolution ==== | ||
* AA may develop gradually into other hematologic disorder which include | * AA may develop gradually into other [[hematologic]] disorder which include<ref name="Brodsky2000" /> | ||
** Paroxysmal nocturnal hemoglobinuria | ** [[Paroxysmal nocturnal hemoglobinuria]] | ||
** Myelodysplastic syndromes | ** [[Myelodysplastic syndromes]] | ||
** Acute myeloid leukemia | ** [[Acute myeloid leukemia, secondary|Acute myeloid leukemia]] | ||
* Clonal evolution in AA can occur due to mutations or cytogenetic abnormalities. | * Clonal evolution in AA can occur due to [[mutations]] or [[Cytogenetics|cytogenetic]] abnormalities. | ||
* The genes that are commonly found to be mutated are | * The genes that are commonly found to be mutated are | ||
| Line 63: | Line 64: | ||
== Genetics == | == Genetics == | ||
Genes involved in the pathogenesis of aplastic anemia include: | [[Genes]] involved in the [[pathogenesis]] of aplastic anemia include:<ref name="Brodsky2000" /> | ||
* HLA-DR15 | * HLA-DR15 | ||
* CD4+ CD25+ FOXP3+ regulatory T cells | * CD4+ CD25+ FOXP3+ regulatory T cells | ||
| Line 70: | Line 71: | ||
* TERC | * TERC | ||
== Associated Conditions == | == Associated Conditions == | ||
* Fanconi's anemia | Aplastic anemia is associated with following conditions:<ref name="Young2002">{{cite journal|last1=Young|first1=Neal S.|title=Acquired Aplastic Anemia|journal=Annals of Internal Medicine|volume=136|issue=7|year=2002|pages=534|issn=0003-4819|doi=10.7326/0003-4819-136-7-200204020-00011}}</ref> | ||
* PNH Paroxysmal Nocturnal Hemoglobinuria | * [[Fanconi's anemia]] | ||
* [[Paroxysmal nocturnal hemoglobinuria|PNH Paroxysmal Nocturnal Hemoglobinuria]] | |||
== Gross Pathology == | == Gross Pathology == | ||
| Line 77: | Line 79: | ||
== Microscopic Pathology == | == Microscopic Pathology == | ||
In aplastic anemia bone marrow microscopy reveals hypo and even acellularity, | In aplastic anemia bone marrow microscopy reveals hypo and even acellularity, [[adipose tissue]] and pale [[stroma]].<ref name="Brodsky2000" /> | ||
==References== | ==References== | ||
Latest revision as of 23:31, 22 October 2018
|
Aplastic anemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Aplastic anemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Aplastic anemia pathophysiology |
|
Risk calculators and risk factors for Aplastic anemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Priyamvada Singh, M.D. [2] Nazia Fuad M.D.
Please help WikiDoc by adding content here. It's easy! Click here to learn about editing.
Overview
Bone marrow is a spongy tissue, found within the spongy or cancellous portions of bones. It is higly vascularized and richly innervated Bone marrow is the primary site of hematopoiesis and is composed of hematopoietic cells, marrow adipose tissue, and stromal cells. The most defenitive feature in pathophysiology of aplastic anemia is loss of hematopoietic stem cells. It may be in the form of hematopoietic failure or immune mediated destruction of bone marrow. Drugs, chemicals, viruses, and different kind of mutations change the immunologic appearance of HSCs resulting in autoimmune destruction of marrow cells. AA may develop gradually into other hematologic disorder which include paroxysmal nocturnal hemoglobinuria [PNH], myelodysplastic syndromes [MDS] and acute myeloid leukemia [AML]). Clonal evolution in AA can occur due to mutations or cytogenetic abnormalities. The genes that are commonly found to be mutated are DMNT3A, ASXL1, BCOR, BCORL1, PIGA.
Pathophysiology
Physiology
The normal physiology of bone marrow can be understood as follows:[1]
- Bone marrow is a spongy tissue, found within the spongy or cancellous portions of bones
- It is higly vascularized and richly innervated
- Bone marrow is the primary site of hematopoiesis.
- It is composed of hematopoietic cells, marrow adipose tissue, and stromal cells.
- Hematopoietic stem cells (HSC) in the bone marrow are the source of all mature cells in the peripheral blood and tissues and are multipotent.
- HSC are recognized and isolated according to their immunophenotype.
- HSCs make a small population within the CD34+/CD38 fraction of bone marrow cells.
- The hematopoiesis is controlled by a various regulatory mechanisms, including growth factors.
- The normal bone marrow structure can be damaged or displaced by aplastic anemia, malignancies or infections.
- This leads to decrease production of blood cells and blood platelets.
.
Pathogenesis
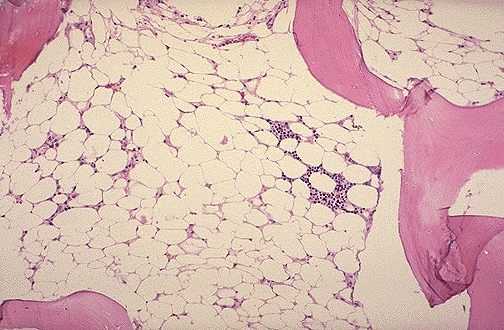 |
The most defenitive feature in pathophysiology of aplastic anemia is loss of hematopoietic stem cells.[2][3]
Pathophysiologic mechanisms that result in loss of HSCs and cause aplastic anemia include:
Hematopoietic Failure
- CD34 cells are almost absent aplastic anemia.
- Progenitor cells capable of forming erythroid, myeloid, and megakaryocytic are greatly reduced.
- The primitive hematopoietic cells which are closely related to stem cells are consistently deficient.
- The white blood cells in aplastic anemia have short telomeres.
- Telomeres are repeats at the end of eukaryotic chromosome and are essential for chromosome protection and complete DNA replication.
Immune-mediated T-cell destruction of marrow
- Drugs, chemicals, viruses, and different kind of mutations change the immunologic appearance of HSCs resulting in autoimmune destruction of marrow cells.[4]
- In patients with acquired aplastic anemia, lymphocytes are responsible for the destruction of the hematopoietic cells.
- These T cells produces an inhibitory factor, interferons , tumor necrosis factor, and interleukin-2, resulting in hematopoietic cell death by apoptosis.
- CD4+CD25+FOXP3+ regulatory T cells are deficient in these patients, similar to what is seen in other autoimmune conditions.
- Deficiency of these regulatory T cells result in increase of T-bet protein levels in T cells, increased interferon (IFN)-γ,2 and stem cell destruction.
- Increased immune response, including tumor necrosis factor -α, IFNγ, and interleukin-6, are also very common in AA patients.
Clonal Evolution
- AA may develop gradually into other hematologic disorder which include[3]
- Clonal evolution in AA can occur due to mutations or cytogenetic abnormalities.
- The genes that are commonly found to be mutated are
- DMNT3A
- ASXL1
- BCOR
- BCORL1
- PIGA
Genetics
Genes involved in the pathogenesis of aplastic anemia include:[3]
- HLA-DR15
- CD4+ CD25+ FOXP3+ regulatory T cells
- STAT3
- TERT
- TERC
Associated Conditions
Aplastic anemia is associated with following conditions:[4]
Gross Pathology
Aplastic anemia does not exhibit any gross pathology
Microscopic Pathology
In aplastic anemia bone marrow microscopy reveals hypo and even acellularity, adipose tissue and pale stroma.[3]
References
- ↑ Hays K (February 1990). "Physiology of normal bone marrow". Semin Oncol Nurs. 6 (1): 3–8. PMID 2406826.
- ↑ Bacigalupo A (2007). "Aplastic anemia: pathogenesis and treatment". Hematology Am Soc Hematol Educ Program: 23–8. doi:10.1182/asheducation-2007.1.23. PMID 18024605.
- ↑ 3.0 3.1 3.2 3.3 Brodsky, R. A. (2000). "Aplastic Anemia: Pathophysiology and Treatment". Journal of the National Cancer Institute. 92 (9): 754–754. doi:10.1093/jnci/92.9.754. ISSN 1460-2105.
- ↑ 4.0 4.1 Young, Neal S. (2002). "Acquired Aplastic Anemia". Annals of Internal Medicine. 136 (7): 534. doi:10.7326/0003-4819-136-7-200204020-00011. ISSN 0003-4819.