Amlodipine besylate and Olmesartan medoxomil: Difference between revisions
No edit summary |
No edit summary |
||
| Line 68: | Line 68: | ||
*Initial therapy with Azor is not recommended in patients ≥75 years old or with hepatic impairment. | *Initial therapy with Azor is not recommended in patients ≥75 years old or with hepatic impairment. | ||
|monitoring=*Monitor renal function periodically in patients receiving olmesartan medoxomil and NSAID therapy. | |||
*Monitor blood pressure, renal function and electrolytes in patients on Azor and other agents that affect the RAS. | |||
*Monitor serum lithium levels during concomitant use. | |||
|alcohol=Alcohol-Amlodipine besylate and Olmesartan medoxomil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Amlodipine besylate and Olmesartan medoxomil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 20:54, 2 September 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
*When pregnancy is detected, discontinue Azor as soon as possible.
|
Overview
Amlodipine besylate and Olmesartan medoxomil is an antihypertensive agent that is FDA approved for the treatment of hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include edema, dizziness, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Azor is indicated for the treatment of hypertension, alone or with other antihypertensive agents , to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. There are no controlled trials demonstrating risk reduction with Azor.
- Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program’s Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
- Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
- Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
- Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
- Azor may also be used as initial therapy in patients who are likely to need multiple antihypertensive agents to achieve their blood pressure goals.
- Patients with moderate or severe hypertension are at relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and should be shaped by considerations such as baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared to monotherapy. Individual blood pressure goals may vary based upon the patient’s risk.
- Data from an 8-week, placebo-controlled, parallel-group factorial study provide estimates of the probability of reaching a blood pressure goal with Azor compared to amlodipine or olmesartan medoxomil monotherapy. The figures below provide estimates of the likelihood of achieving the targeted systolic or diastolic blood pressure goals with Azor 10/40 mg compared with amlodipine or olmesartan medoxomil monotherapy, based upon baseline systolic or diastolic blood pressure. The curve of each treatment group was estimated by logistic regression modeling from all available data of that treatment group. The right tail of each curve is less reliable because of small numbers of subjects with high baseline blood pressures.
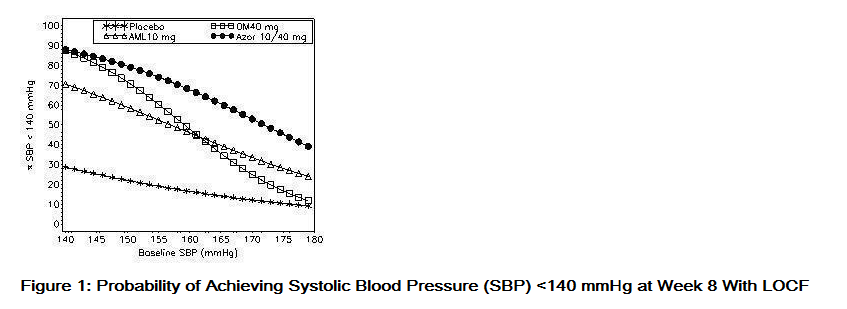
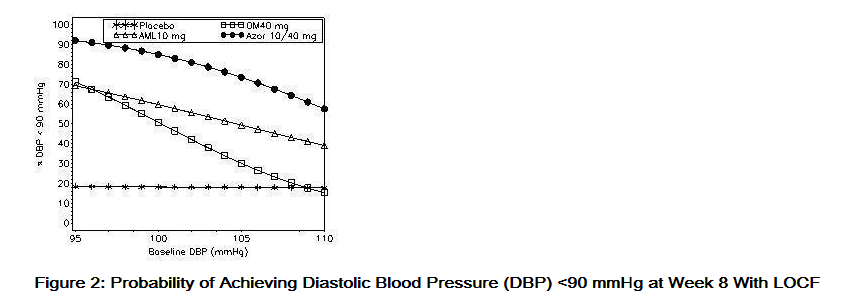
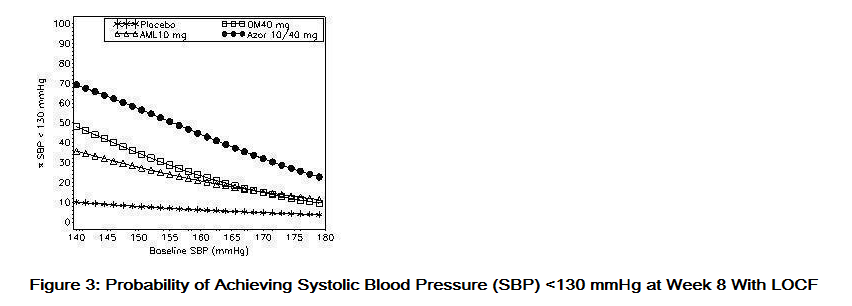
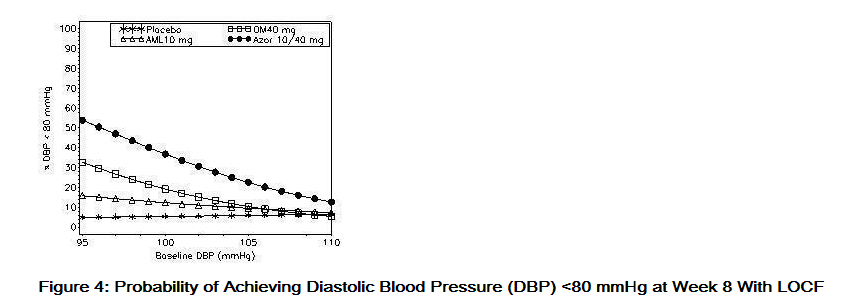
- The figures above provide an approximation of the likelihood of reaching a targeted blood pressure goal (e.g., Week 8 SBP <140 mmHg or <130 mmHg or a DBP <90 mmHg or <80 mmHg) for the high-dose treatment groups evaluated in the study. Azor 5/20 mg, the lowest dose combination treatment group, increases the probability of reaching blood pressure goal compared with the highest dose monotherapies, amlodipine 10 mg and olmesartan medoxomil 40 mg.
- For example, a patient with a baseline blood pressure of 160/100 mmHg has about a 48% likelihood of achieving a goal of <140 mmHg (systolic) and a 51% likelihood of achieving a goal of <90 mmHg (diastolic) on monotherapy with olmesartan medoxomil 40 mg, and about a 46% likelihood of achieving a goal of <140 mmHg (systolic) and a 60% likelihood of achieving a goal of <90 mmHg (diastolic) on monotherapy with amlodipine 10 mg. The likelihood of achieving these same goals increases to 63% (systolic) and 71% (diastolic) on Azor 5/20 mg, and to 68% (systolic) and 85% (diastolic) on Azor 10/40 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Amlodipine besylate and Olmesartan medoxomil in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Amlodipine besylate and Olmesartan medoxomil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Amlodipine besylate and Olmesartan medoxomil in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Amlodipine besylate and Olmesartan medoxomil in pediatric patients.
Contraindications
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Contraindications in the drug label.
Warnings
|
WARNING: FETAL TOXICITY
See full prescribing information for complete Boxed Warning.
*When pregnancy is detected, discontinue Azor as soon as possible.
|
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Warnings' in the drug label.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Amlodipine besylate and Olmesartan medoxomil in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Amlodipine besylate and Olmesartan medoxomil in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Amlodipine besylate and Olmesartan medoxomil during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil in geriatric settings.
Gender
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Amlodipine besylate and Olmesartan medoxomil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Amlodipine besylate and Olmesartan medoxomil in patients who are immunocompromised.
Administration and Monitoring
Administration
General Considerations:
- The side effects of olmesartan medoxomil are generally rare and apparently independent of dose. Those of amlodipine are generally dose-dependent (mostly edema).
- Maximum antihypertensive effects are attained within 2 weeks after a change in dose.
- Azor may be taken with or without food.
- Azor may be administered with other antihypertensive agents.
- Dosage may be increased after 2 weeks. The maximum recommended dose of Azor is 10/40 mg.
Replacement Therapy:
- Azor may be substituted for its individually titrated components.
- When substituting for individual components, the dose of one or both of the components can be increased if blood pressure control has not been satisfactory.
Add-on Therapy:
- Azor may be used to provide additional blood pressure lowering for patients not adequately controlled with amlodipine (or another dihydropyridine calcium channel blocker) alone or with olmesartan medoxomil (or another angiotensin receptor blocker) alone.
Initial Therapy:
- The usual starting dose of Azor is 5/20 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum dose of one 10/40 mg tablet once daily as needed to control blood pressure.
- Initial therapy with Azor is not recommended in patients ≥75 years old or with hepatic impairment.
Monitoring
- Monitor renal function periodically in patients receiving olmesartan medoxomil and NSAID therapy.
- Monitor blood pressure, renal function and electrolytes in patients on Azor and other agents that affect the RAS.
- Monitor serum lithium levels during concomitant use.
IV Compatibility
There is limited information regarding the compatibility of Amlodipine besylate and Olmesartan medoxomil and IV administrations.
Overdosage
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Mechanism of Action in the drug label.
Structure
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Structure in the drug label.
Pharmacodynamics
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Clinical Studies in the drug label.
How Supplied
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil How Supplied in the drug label.
Storage
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Amlodipine besylate and Olmesartan medoxomil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Amlodipine besylate and Olmesartan medoxomil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Amlodipine besylate and Olmesartan medoxomil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Amlodipine besylate and Olmesartan medoxomil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.