Alkane
Overview
Alkanes, also known as Paraffins, are chemical compounds that consist only of the elements carbon (C) and hydrogen (H) (i.e. hydrocarbons), where each of these atoms are linked together exclusively by single bonds (i.e. they are saturated compounds) without any cyclic structure (i.e. loops). Alkanes belong to a homologous series of organic compounds in which the members differ by a constant relative atomic mass of 14.
Each carbon atom must have 4 bonds (either C-H or C-C bonds), and each hydrogen atom must be joined to a carbon atom (H-C bonds). A series of linked carbon atoms is known as the carbon skeleton or carbon backbone. Typically the number of carbon atoms is often used to define the size of the alkane (e.g. C2-alkane).
An alkyl group is a functional group or side chain which, like an alkane, consists solely of singly bonded carbon and hydrogen atoms, for example a methyl or ethyl group.
Saturated hydrocarbons can be linear (general formula CnH2n+2) where the carbon atoms are joined in a snake-like structure, branched (general formula CnH2n+2, n>3) where the carbon backbone splits off in one or more directions, or cyclic (general formula CnH2n, n>2) where the carbon backbone is linked so as to form a loop. According to the definition by IUPAC, the former two are alkanes, while the third group is called cycloalkanes.[1] In other words, saturated hydrocarbons are divided into alkanes and cycloalkanes, depending on whether or not they have cyclic structures, and technically, cycloalkanes are not alkanes. However, cycloalkanes are sometimes called cyclic alkanes, confusingly, when "real" alkanes are called acyclic alkanes. Saturated hydrocarbons can also combine any of the linear, cyclic (e.g. polycyclic) and branching structures, and they are still alkanes (no general formula) as long as they are acyclic (i.e. having no loops).
The simplest possible alkane (the parent molecule) is methane, CH4. There is no limit to the number of carbon atoms that can be linked together, the only limitation being that the molecule is acyclic, is saturated, and is a hydrocarbon. Saturated oils and waxes are examples of larger alkanes where the number of carbons in the carbon backbone tends to be greater than 10.
Alkanes are not very reactive and have little biological activity. Alkanes can be viewed as a molecular scaffold upon which can be hung the interesting biologically active/reactive portions (functional groups) of the molecule.
Isomerism
Alkanes with more than three carbon atoms can be arranged in a multiple number of ways, forming different structural isomers. An isomer is like a chemical anagram, in which the atoms of a chemical compound are arranged or joined together in a different order. The simplest isomer of an alkane is the one in which the carbon atoms are arranged in a single chain with no branches. This isomer is sometimes called the n-isomer (n for "normal", although it is not necessarily the most common). However the chain of carbon atoms may also be branched at one or more points. The number of possible isomers increases rapidly with the number of carbon atoms. For example:
- C1: 1 isomer — methane
- C2: 1 isomer — ethane
- C3: 1 isomers — propane
- C4: 2 isomers — n-butane, isobutane
- C12: 355 isomers
- C32: 27,711,253,769 isomers
- C60: 22,158,734,535,770,411,074,184 isomers
In addition to these isomers, the chain of carbon atoms may form one or more loops. Such compounds are called cycloalkanes.
Nomenclature
The IUPAC nomenclature (systematic way of naming compounds) for alkanes is based on identifying hydrocarbon chains. Unbranched, saturated hydrocarbon chains are named systematically with a Greek numerical prefix denoting the number of carbons and the suffix "-ane".[2]
August Wilhelm von Hofmann suggested systematizing nomenclature by using the whole sequence of vowels a, e, i, o and u to create suffixes -ane, -ene, -ine (or -yne), -one, -une, for the hydrocarbons. The first three name hydrocarbons with single, double and triple bonds. "-one" represents a ketone. "-ol" represents an alcohol or OH group. "-oxy-" means an ether and refers to oxygen between two carbons, so that methoxy-methane is the IUPAC name for dimethyl ether.
It is difficult or impossible to find compounds with more than one IUPAC name. This is because shorter chains attached to longer chains are prefixes and the convention includes brackets. Numbers in the name, referring to which carbon a group is attached to, should be as low as possible, so that 1- is implied and usually omitted from names of organic compounds with only one side-group. "1-" is implied in Nitro-octane. Symmetric compounds will hav two ways of arriving at the same name.
Linear alkanes
Straight-chain alkanes are sometimes indicated by the prefix n- (for normal) where a non-linear isomer exists. Although this is not strictly necessary, the usage is still common in cases where there is an important difference in properties between the straight-chain and branched-chain isomers: e.g. n-hexane or 2- or 3-methylpentane.
The first four members of the series (in terms of number of carbon atoms) are named as follows:
Alkanes with five or more carbon atoms are named by adding the suffix -ane to the appropriate numerical multiplier[3] with elision of a terminal -a- from the basic numerical term. Hence, pentane, C5H12; hexane, C6H14; heptane, C7H16; octane, C8H18; etc. For a more complete list, see List of alkanes.
Branched alkanes
Simple branched alkanes often have a common name using a prefix to distinguish them from linear alkanes, for example n-pentane, isopentane, and neopentane.
Alternately, IUPAC naming conventions can be used to produce a systematic name.
The key steps in the naming of more complicate branched alkanes are as follows:[4]
- Identify the longest linear chain of carbon atoms.
- Name this longest root chain using standard naming rules
- Name each side chain by changing the suffix of the name of the alkane from "-ane" to "-yl"
- Number the root chain so that sum total of the numbers assigned to each side group will be as low as possible.
- Number and name the side chains before the name of the root chain
- If there are multiple side chains of the same type, use prefixes such as "di-" and "tri-" to indicate it as such, and number each one.
| Common name | n-pentane | isopentane | neopentane |
|---|---|---|---|
| IUPAC name | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Structure | 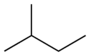 |
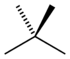
|
Cyclic alkanes
So-called cyclic alkanes are technically not alkanes, but cycloalkanes. They are hydrocarbons just like alkanes, but are containing one or more rings.
Simple cycloalkanes have a prefix "cyclo-" to distinguish them from alkanes. Cycloalkanes are named as per their acyclic counterparts with respect to the number of carbon atoms, e.g. cyclopentane (C5H10) is a cycloalkane with 5 carbon atoms just like pentane (C5H12), but they are joined up in a five-membered ring. Similarly, propane and cyclopropane, butane and cyclobutane, etc.
Substituted cycloalkanes are named similar to substituted alkanes — the cycloalkane ring is stated, and the substituents are according to their position on the ring, with the numbering decided by Cahn-Ingold-Prelog rules.[3]
Trivial names
The trivial (non-systematic) name for alkanes is "paraffins". Collectively, alkanes are known as the paraffin series. Trivial names for compounds are usually historical artifacts. They were coined before the development of systematic names, and have been retained due to familiar usage in industry. Cycloalkanes are also called naphthenes.
The term paraffins almost certainly stems from the petrochemical industry. Branched-chain alkanes are called isoparaffins. The use of the term "paraffin" is a general term and often does not distinguish between a pure compounds and mixtures of isomers with the same chemical formula (i.e. like a chemical anagram) e.g. pentane and isopentane.
- Examples
The following trivial names are retained in the IUPAC system:
- isobutane for 2-methylpropane
- isopentane for 2-methylbutane
- neopentane for 2,2-dimethylpropane
Occurrence
Occurrence of alkanes in the Universe

Alkanes form a significant portion of the atmospheres of the outer gas planets such as Jupiter (0.1% methane, 0.0002% ethane), Saturn (0.2% methane, 0.0005% ethane), Uranus (1.99% methane, 0.00025% ethane) and Neptune (1.5% methane, 1.5 ppm ethane). Titan (1.6% methane), a satellite of Saturn, was examined by the Huygens probe which indicate that Titan's atmosphere periodically rains liquid methane onto the moon's surface.[5] Also on Titan, a methane-spewing volcano was spotted and this volcanism is believed to be a significant source of the methane in the atmosphere. There also appear to be Methane/Ethane lakes near the north polar regions of Titan, as discovered by Cassini's radar imaging. Methane and ethane have also been detected in the tail of the comet Hyakutake. Chemical analysis showed that the abundances of ethane and methane were roughly equal, which is thought to imply that its ices formed in interstellar space, away from the Sun, which would have evaporated these volatile molecules.[6]. Alkanes have also been detected in meteorites such as carbonaceous chondrites.
Occurrence of alkanes on Earth
Traces of methane gas (about 0.0001% or 1 ppm) occur in the Earth's atmosphere, produced primarily by organisms such as Archaea, found for example in the gut of cows.

The most important commercial sources for alkanes are natural gas and oil.[7] Natural gas contains primarily methane and ethane, with some propane and butane: oil is a mixture of liquid alkanes and other hydrocarbons. These hydrocarbons were formed when dead marine animals and plants (zooplankton and phytoplankton) died and sank to the bottom of ancient seas and were covered with sediments in an anoxic environment and converted over many millions of years at high temperatures and high pressure to their current form. Natural gas resulted thereby for example from the following reaction:
- C6H12O6 → 3CH4 + 3CO2
These hydrocarbons collected in porous rocks, located beneath an impermeable cap rock and so are trapped. Unlike methane, which is constantly reformed in large quantities, higher alkanes (alkanes with 9 or more carbon atoms) rarely develop to a considerable extent in nature. These deposits e.g. (oil fields) have formed over millions of years and once exhausted can not be readily replaced. The depletion of these hydrocarbons is the basis for what is known as the energy crisis.
Solid alkanes are known as tars and are formed when more volatile alkanes such as gases and oil evaporate from hydrocarbon deposits. One of the largest natural deposits of solid alkanes is in the asphalt lake known as the Pitch Lake in Trinidad and Tobago.
Methane is also present in what is called biogas, produced by animals and decaying matter, which is a possible renewable energy source.
Alkanes have a low solubility in water, so the content in the oceans is negligible: however, at high pressures and low temperatures (such as at the bottom of the oceans), methane can co-crystallize with water to form a solid methane hydrate. Although this cannot be commercially exploited at the present time, the amount of combustible energy of the known methane hydrate fields exceeds the energy content of all the natural gas and oil deposits put together;methane extracted from methane hydrate is considered therefore a candidate for future fuels.
Biological occurrence
Although alkanes occur in nature in various way, they do not rank biologically among the essential materials. Cycloalkanes with 14 to 18 carbon atoms occur in musk, extracted from deer of the family Moschidae. All further information refers to (acyclic) alkanes.
- Bacteria and archaea

Certain types of bacteria can metabolise alkanes: they prefer even-numbered carbon chains as they are easier to degrade than odd-numbered chains.
On the other hand certain archaea, the methanogens, produce large quantities of methane by the metabolism of carbon dioxide or other oxidised organic compounds. The energy is released by the oxidation of hydrogen:
- CO2 + 4H2 → CH4 + 2H2O
Methanogens are also the producers of marsh gas in wetlands, and release about two billion tonnes of methane per year — the atmospheric content of this gas is produced nearly exclusively by them. The methane output of cattle and other herbivores, which can release up to 150 litres per day, and of termites, is also due to methanogens. They also produce this simplest of all alkanes in the intestines of humans. Methanogenic archaea are hence at the end of the carbon cycle, with carbon being released back into the atmosphere after having been fixed by photosynthesis. It is probable that our current deposits of natural gas were formed in a similar way.
- Fungi and plants

Alkanes also play a role, if a minor role, in the biology of the three eukaryotic groups of organisms: fungi, plants and animals. Some specialised yeasts, e.g. Candida tropicale, Pichia sp., Rhodotorula sp., can use alkanes as a source of carbon and/or energy. The fungus Amorphotheca resinae prefers the longer-chain alkanes in aviation fuel, and can cause serious problems for aircraft in tropical regions.
In plants it is the solid long-chain alkanes that are found; they form a firm layer of wax, the cuticle, over areas of the plant exposed to the air. This protects the plant against water loss, while preventing the leaching of important minerals by the rain. It is also a protection against bacteria, fungi and harmful insects — the latter sink with their legs into the soft waxlike substance and have difficulty moving. The shining layer on fruits such as apples consists of long-chain alkanes. The carbon chains are usually between twenty and thirty carbon atoms in length and are made by the plants from fatty acids. The exact composition of the layer of wax is not only species-dependent, but changes also with the season and such environmental factors as lighting conditions, temperature or humidity.
- Animals
Alkanes are found in animal products, although they are less important than unsaturated hydrocarbons. One example is the shark liver oil, which is approximately 14% pristane (2,6,10,14-tetramethylpentadecane, C19H40). Their occurrence is more important in pheromones, chemical messenger materials, on which above all insects are dependent for communication. With some kinds, as the support beetle Xylotrechus colonus, primarily pentacosane (C25H52), 3-methylpentaicosane (C26H54) and 9-methylpentaicosane (C26H54), they are transferred by body contact. With others like the tsetse fly Glossina morsitans morsitans, the pheromone contains the four alkanes 2-methylheptadecane (C18H38), 17,21-dimethylheptatriacontane (C39H80), 15,19-dimethylheptatriacontane (C39H80) and 15,19,23-trimethylheptatriacontane (C40H82), and acts by smell over longer distances, a useful characteristic for pest control.
Ecological relations

One example, in which both plant and animal alkanes play a role, is the ecological relationship between the sand bee (Andrena nigroaenea) and the early spider orchid (Ophrys sphegodes); the latter is dependent for pollination on the former. Sand bees use pheromones in order to identify a mate; in the case of A. nigroaenea, the females emit a mixture of tricosane (C23H48), pentacosane (C25H52) and heptacosane (C27H56) in the ratio 3:3:1, and males are attracted by specifically this odour. The orchid takes advantage of this mating arrangement to get the male bee to collect and disseminate its pollen; parts of its flower not only resemble the appearance of sand bees, but also produce large quantities of the three alkanes in the same ratio as female sand bees. As a result numerous males are lured to the blooms and attempt to copulate with their imaginary partner: although this endeavour is not crowned with success for the bee, it allows the orchid to transfer its pollen, which will be dispersed after the departure of the frustrated male to different blooms.
Production
Petroleum refining

As stated earlier, the most important source of alkanes is natural gas and crude oil.[7] Alkanes are separated in an oil refinery by fractional distillation and processed into many different products
Fischer-Tropsch
The Fischer-Tropsch process is a method to synthesize liquid hydrocarbons, including alkanes, from carbon monoxide and hydrogen. This method is used to produce substitutes for petroleum distillates.
Laboratory preparation
There is usually little need for alkanes to be synthesized in the laboratory, since they are usually commercially available. Also, alkanes are generally non-reactive chemically or biologically, and do not undergo functional group interconversions cleanly. When alkanes are produced in the laboratory, it is often a side product of a reaction. For example, the use of n-butyllithium as a strong base gives the conjugate acid, n-butane as a side product:
- C4H9Li + H2O → C4H10 + LiOH
However, at times it may be desirable to make a portion of a molecule into an alkane like functionality (alkyl group) using the above or similar methods. For example an ethyl group is an alkyl group, when this is attached to a hydroxy group it gives ethanol, which is not an alkane. To do so, the best-known methods are hydrogenation of alkenes:
- RCH=CH2 + H2 → RCH2CH3 (R = alkyl)
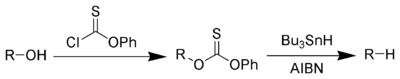
Alkanes or alkyl groups can also be prepared directly from alkyl halides in the Corey-House-Posner-Whitesides reaction. The Barton-McCombie deoxygenation[8][9] removes hydroxyl groups from alcohols e.g.
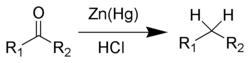
and the Clemmensen reduction[10][11][12][13] removes carbonyl groups from aldehydes and ketones to form alkanes or alkyl-substituted compounds e.g.:
Applications
The applications of a certain alkane can be determined quite well according to the number of carbon atoms. The first four alkanes are used mainly for heating and cooking purposes, and in some countries for electricity generation. Methane and ethane are the main components of natural gas; they are normally stored as gases under pressure. It is however easier to transport them as liquids: this requires both compression and cooling of the gas.
Propane and butane can be liquefied at fairly low pressures, and are well known as liquified petroleum gas (LPG). Propane, for example, is used in the propane gas burner, butane in disposable cigarette lighters. The two alkanes are used as propellants in aerosol sprays.
From pentane to octane the alkanes are reasonably volatile liquids. They are used as fuels in internal combustion engines, as they vaporise easily on entry into the combustion chamber without forming droplets which would impair the unifomity of the combustion. Branched-chain alkanes are preferred, as they are much less prone to premature ignition which causes knocking than their straight-chain homologue. This propensity to premature ignition is measured by the octane rating of the fuel, where 2,2,4-trimethylpentane (isooctane) has an arbitrary value of 100 and heptane has a value of zero. Apart from their use as fuels, the middle alkanes are also good solvents for nonpolar substances.
Alkanes from nonane to, for instance, hexadecane (an alkane with sixteen carbon atoms) are liquids of higher viscosity, less and less suitable for use in gasoline. They form instead the major part of diesel and aviation fuel. Diesel fuels are characterised by their cetane number, cetane being an old name for hexadecane. However, the higher melting points of these alkanes can cause problems at low temperatures and in polar regions, where the fuel becomes too thick to flow correctly.
Alkanes from hexadecane upwards form the most important components of fuel oil and lubricating oil. In latter function they work at the same time as anti-corrosive agents, as their hydrophobic nature means that water cannot reach the metal surface. Many solid alkanes find use as paraffin wax, for example in candles. This should not be confused however with true wax, which consists primarily of esters.
Alkanes with a chain length of approximately 35 or more carbon atoms are found in bitumen, used for example in road surfacing. However, the higher alkanes have little value and are usually split into lower alkanes by cracking.
Some synthetic polymers such as polyethylene and polypropylene are alkanes with chains containing hundreds of thousands of carbon atoms. These materials are used in innumerable applications and billions of kilograms of these materials are made and used each year.
Physical properties
Boiling point
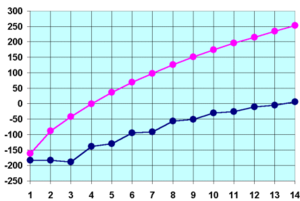
Alkanes experience inter-molecular van der Waals forces. Stronger inter-molecular van der Waals forces give rise to greater boiling points of alkanes.[7]
There are two determinants for the strength of the van der Waals forces:
- the number of electrons surrounding the molecule, which increase with the alkane's molecular weight
- the surface area of the molecule
Under standard conditions, from CH4 to C4H10 alkanes are gaseous; from C5H12 to C17H36 they are liquids; and after C18H38 they are solids. As the boiling point of alkanes is primarily determined by weight, it should not be a surprise that the boiling point has almost a linear relationship with the size (molecular weight) of the molecule. As a rule of thumb, the boiling point rises 20 - 30 °C for each carbon added to the chain; this rule applies to other homologous series.[7]
A straight-chain alkane will have a boiling point higher than a branched-chain alkane due to the greater surface area in contact, thus the greater van der Waals forces, between adjacent molecules. For example, compare isobutane and n-butane which boil at -12 and 0 °C, and 2,2-dimethylbutane and 2,3-dimethylbutane which boil at 50 and 58 °C respectively.[7] For the latter case, two molecules 2,3-dimethylbutane can "lock" into each other better than the cross-shaped 2,2-dimethylbutane, hence the greater van der Waals forces.
On the other hand, cycloalkanes tend to have higher boiling points than their linear counterparts due to the locked conformations of the molecules which give a plane of intermolecular contact.
Melting point
The melting points of the alkanes follow a similar trend to boiling points for the same reason as outlined above. That is, (all other things being equal) the larger the molecule the higher the melting point. There is one significant difference between boiling points and melting points. Solids have more ridged and fixed structure than liquids. This rigid structure requires energy to break down. Thus the stronger better put together solid structures will require more energy to break apart. For alkanes, this can be seen from the graph above (i.e. the blue line). The odd numbered alkanes have a lower trend in melting points that even numbered alkanes. This is because even numbered alkanes pack well in the solid phase, forming a well organised structure which requires more energy to break apart. The odd number alkanes pack less well and so the "looser" organised solid packing structure requires less energy to break apart. [14]
The melting points of branched-chain alkanes can be either higher or lower than those of the corresponding straight-chain alkanes, again this depends on the ability of the alkane in question to packing well in the solid phase: this is particularly true for isoalkanes (2-methyl isomers), which often have melting points higher than those of the linear analogues.
Conductivity
Alkanes do not conduct electricity, nor are they substantially polarized by an electric field. For this reason they do not form hydrogen bonds and are insoluble in polar solvents such as water. Since the hydrogen bonds between individual water molecules are aligned away from an alkane molecule, the coexistence of an alkane and water leads to an increase in molecular order (a reduction in entropy). As there is no significant bonding between water molecules and alkane molecules, the second law of thermodynamics suggests that this reduction in entropy should be minimised by minimising the contact between alkane and water: alkanes are said to be hydrophobic in that they repel water.
Their solubility in nonpolar solvents is relatively good, a property which is called lipophilicity. Different alkanes are, for example, miscible in all proportions among themselves.
The density of the alkanes usually increases with increasing number of carbon atoms, but remains less than that of water. Hence, alkanes form the upper layer in an alkane-water mixture.
Molecular geometry
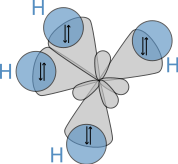
The molecular structure of the alkanes directly affects their physical and chemical characteristics. It is derived from the electron configuration of carbon, which has four valence electrons. The carbon atoms in alkanes are always sp3 hybridised, that is to say that the valence electrons are said to be in four equivalent orbitals derived from the combination of the 2s orbital and the three 2p orbitals. These orbitals, which have identical energies, are arranged spatially in the form of a tetrahedron, the angle of cos−1(−⅓) ≈ 109.47° between them.
Bond lengths and bond angles
An alkane molecule has only C – H and C – C single bonds. The former result from the overlap of a sp³-orbital of carbon with the 1s-orbital of a hydrogen; the latter by the overlap of two sp³-orbitals on different carbon atoms. The bond lengths amount to 1.09×10−10 m for a C – H bond and 1.54×10−10 m for a C – C bond.
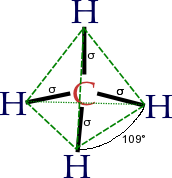
The spatial arrangement of the bonds is similar to that of the four sp³-orbitals — they are tetrahedrally arranged, with an angle of 109.47° between them. Structural formulae which represent the bonds as being at right angles to one another, while both common and useful, do not correspond with the reality.
Conformation
The structural formula and the bond angles are not usually sufficient to completely describe the geometry of a molecule. There is a further degree of freedom for each carbon – carbon bond: the torsion angle between the atoms or groups bound to the atoms at each end of the bond. The spatial arrangement described by the torsion angles of the molecule is known as its conformation.
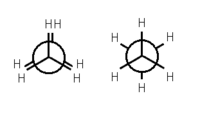
Ethane forms the simplest case for studying the conformation of alkanes, as there is only one C – C bond. If one looks down the axis of the C – C bond, then one will see the so-called Newman projection. The hydrogen atoms on both the front and rear carbon atoms have an angle of 120° between them, resulting from the projection of the base of the tetrahedron onto a flat plane. However, the torsion angle between a given hydrogen atom attached to the front carbon and a given hydrogen atom attached to the rear carbon can vary freely between 0° and 360°. This is a consequence of the free rotation about a carbon – carbon single bond. Despite this apparent freedom, only two limiting conformations are important: eclipsed conformation and staggered conformation.
The two conformations, also known as rotamers, differ in energy: The staggered conformation is 12.6 kJ/mol lower in energy (more stable) than the eclipsed conformation (the least stable).
This difference in energy between the two conformations, known as the torsion energy, is low compared to the thermal energy of an ethane molecule at ambient temperature. There is constant rotation about the C-C bond. The time taken for an ethane molecule to pass from one staggered conformation to the next, equivalent to the rotation of one CH3-group by 120° relative to the other, is of the order of 10−11 seconds.
The case of higher alkanes is more complex but based on similar principles, with the antiperiplanar conformation always being the most favoured around each carbon-carbon bond. For this reason, alkanes are usually shown in a zigzag arrangement in diagrams or in models. The actual structure will always differ somewhat from these idealised forms, as the differences in energy between the conformations are small compared to the thermal energy of the molecules: alkane molecules have no fixed structural form, whatever the models may suggest.
Spectroscopic properties
Virtually all organic compounds contain carbon – carbon and carbon – hydrogen bonds, and so show some of the features of alkanes in their spectra. Alkanes are notable for having no other groups, and therefore for the absence of other characteristic spectroscopic features.
Infrared spectroscopy
The carbon – hydrogen stretching mode gives a strong absorption between 2850 and 2960 nanometres, while the carbon – carbon stretching mode absorbs between 800 and 1300 nm. The carbon – hydrogen bending modes depend on the nature of the group: methyl groups show bands at 1450 nm and 1375 nm, while methylene groups show bands at 1465 nm and 1450 nm. Carbon chains with more than four carbon atoms show a weak absorption at around 725 nm.
NMR spectroscopy
The proton resonances of alkanes are usually found at δH = 0.5 – 1.5. The carbon-13 resonances depend on the number of hydrogen atoms attached to the carbon: δC = 8 – 30 (primary, methyl, -CH3), 15 – 55 (secondary, methylene, -CH2-), 20 – 60 (tertiary, methyne, C-H) and quaternary. The carbon-13 resonance of quaternary carbon atoms is characteristically weak, due to the lack of Nuclear Overhauser effect and the long relaxation time, and can be missed in weak samples, or sample that have not been run for a sufficiently long time.
Mass spectrometry
Alkanes have a high ionisation energy, and the molecular ion is usually weak. The fragmentation pattern can be difficult to interpret, but, in the case of branched chain alkanes, the carbon chain is preferentially cleaved at tertiary or quaternary carbons due to the relative stability of the resulting free radicals. The fragment resulting from the loss of a single methyl group (M−15) is often absent, and other fragment are often spaced by intervals of fourteen mass units, corresponding to sequential loss of CH2-groups.
Chemical properties
Alkanes generally show a relatively low reactivity, because their C bonds are relatively stable and cannot be easily broken. Unlike most other organic compounds, they possess no functional groups.
They react only very poorly with ionic or other polar substances. The acid dissociation constant (pKa) values of all alkanes are above 60, hence they are practically inert to acids and bases (see: carbon acids). This inertness is the source of the term paraffins (with the meaning here of "lacking affinity"). In crude oil the alkane molecules have remained chemically unchanged for millions of years.
However redox reactions of alkanes, in particular with oxygen and the halogens, are possible as the carbon atoms are in a strongly reduced condition; in the case of methane, the lowest possible oxidation state for carbon (−4) is reached. Reaction with oxygen leads to combustion without any smoke; with halogens, substitution. In addition, alkanes have been shown to interact with, and bind to, certain transition metal complexes in (See: carbon-hydrogen bond activation).
Free radicals, molecules with unpaired electrons, play a large role in most reactions of alkanes, such as cracking and reformation where long-chain alkanes are converted into shorter-chain alkanes and straight-chain alkanes into branched-chain isomers.
In highly branched alkanes, the bond angle may differ significantly from the optimal value (109.5°) in order to allow the different groups sufficient space. This causes a tension in the molecule, known as steric hindrance, and can substantially increase the reactivity.
Reactions with oxygen
All alkanes react with oxygen in a combustion reaction, although they become increasingly difficult to ignite as the number of carbon atoms increases. The general equation for complete combustion is:
- CnH2n+2 + (1.5n+0.5)O2 → (n+1)H2O + nCO2
In the absence of sufficient oxygen, carbon monoxide or even soot can be formed, as shown below:
for example methane:
- 2CH4 + 3O2 → 2CO + 4H2O
- CH4 + O2 → C + 2H2O
See the alkane heat of formation table for detailed data.
The standard enthalpy change of combustion, ΔcHo, for alkanes increases by about 650 kJ/mol per CH2 group. Branched-chain alkanes have lower values of ΔcHo than straight-chain alkanes of the same number of carbon atoms, and so can be seen to be somewhat more stable.
Reactions with halogens
Alkanes react with halogens in a so-called free radical halogenation reaction. The hydrogen atoms of the alkane are progressively replaced by halogen atoms. Free radicals are the reactive species which participate in the reaction, which usually leads to a mixture of products. The reaction is highly exothermic, and can lead to an explosion.
These reactions are an important industrial route to halogenated hydrocarbons. There are three steps:
- Initiation the halogen radicals form by homolysis. Usually, energy in the form of heat or light is required.
- Chain reaction then takes place — the halogen radical abstracts a hydrogen from the alkane to give an alkyl radical. This reacts further.
- 'Chain termination where step the radicals recombine.
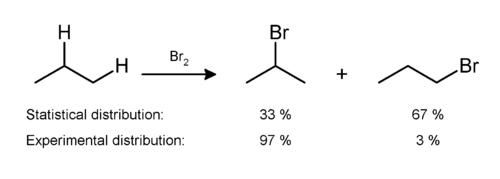
Experiments have shown that all halogenation produces a mixture of all possible isomers, indicating that all hydrogen atoms are susceptible to reaction. The mixture produced, however, is not a statistical mixture: secondary and tertiary hydrogen atoms are preferentially replaced due to the greater stability of secondary and tertiary free radicals. An example can be seen in the monobromination of propane:[7]
Cracking
Cracking breaks larger molecules into smaller ones. This can be done with a thermal or catalytic method. The thermal cracking process follows a homolytic mechanism, that is, bonds break symmetrically and thus pairs of free radicals are formed. The catalytic cracking process involves the presence of acid catalysts (usually solid acids such as silica-alumina and zeolites) which promote a heterolytic (asymmetric) breakage of bonds yielding pairs of ions of opposite charges, usually a carbocation and the very unstable hydride anion. Carbon-localized free radicals and cations are both highly unstable and undergo processes of chain rearrangement, C-C scission in position beta (i.e., cracking) and intra- and intermolecular hydrogen transfer or hydride transfer. In both types of processes, the corresponding reactive intermediates (radicals, ions) are permanently regenerated, and thus they proceed by a self-propagating chain mechanism. The chain of reactions is eventually terminated by radical or ion recombination.
Here is an example of cracking with butane CH3-CH2-CH2-CH3
- 1st possibility (48%): breaking is done on the CH3-CH2 bond.
CH3* / *CH2-CH2-CH3
after a certain number of steps, we will obtain an alkane and an alkene: CH4 + CH2=CH-CH3
- 2nd possibility (38%): breaking is done on the CH2-CH2 bond.
CH3-CH2* / *CH2-CH3
after a certain number of steps, we will obtain an alkane and an alkene from different types: CH3-CH3 + CH2=CH2
- 3rd possibility (14%): breaking of a C-H bond
after a certain number of steps, we will obtain an alkene and hydrogen gas: CH2=CH-CH2-CH3 + H2
Isomerization and reformation
Isomerization and reformation are processes in which straight-chain alkanes are heated in the presence of a platinum catalyst. In isomerization, the alkanes become branched-chain isomers. In reformation, the alkanes become cycloalkanes or aromatic hydrocarbons, giving off hydrogen as a by-product. Both of these processes raise the octane number of the substance.
Other reactions
Alkanes will react with steam in the presence of a nickel catalyst to give hydrogen. Alkanes can by chlorosulfonated and nitrated, although both reactions require special conditions. The fermentation of alkanes to carboxylic acids is of some technical importance. In the Reed reaction, sulfur dioxide, chlorine and light convert hydrocarbons to sulfonyl chlorides.
Hazards
Methane is explosive when mixed with air (1 – 8% CH4) and is a strong greenhouse gas: other lower alkanes can also form explosive mixtures with air. The lighter liquid alkanes are highly flammable, although this risk decreases with the length of the carbon chain. Pentane, hexane, heptane and octane are classed as dangerous for the environment and harmful. The straight chain isomer of hexane is a neurotoxin, and therefore rarely used commercially.
See also
Further reading
Template:Alkanes Template:Functional Groups Template:BranchesofChemistry
References
- ↑ Template:GoldBookRef
- ↑ IUPAC, Commission on Nomenclature of Organic Chemistry (1993). "R-2.2.1: Hydrocarbons". A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993). Blackwell Scientific. Retrieved 2007-02-12.
- ↑ 3.0 3.1 William Reusch. "Nomenclature - Alkanes". Virtual Textbook of Organic Chemistry.
- ↑ William Reusch. "Examples of the IUPAC Rules in Practice". Virtual Textbook of Organic Chemistry.
- ↑ Titan: Arizona in an Icebox?, Emily Lakdawalla, 2004-01-21, verified 2005-03-28
- ↑ Mumma, M.J. (1996). "Detection of Abundant Ethane and Methane, Along with Carbon Monoxide and Water, in Comet C/1996 B2 Hyakutake: Evidence for Interstellar Origin". Science. 272: 1310. doi:10.1126/science.272.5266.1310. Unknown parameter
|co-authors=ignored (help) - ↑ 7.0 7.1 7.2 7.3 7.4 7.5 R. T. Morrison, R. N. Boyd. Organic Chemistry (6th ed.). New Jersey: Prentice Hall. ISBN 0-13-643669-2.
- ↑ Barton, D. H. R.; McCombie, S. W. J. Chem. Soc., Perkin Trans. 1 1975, 16, 1574-1585
- ↑ Crich, D.; Quintero, L. Chem. Rev. 1989, 89, 1413-1432.
- ↑ Martin, E. L. Org. React. 1942, 1, 155. (Review)
- ↑ Buchanan, J. G. St. C.; Woodgate, P. D. Quart. Rev. 1969, 23, 522. (Review)
- ↑ Vedejs, E. Org. React. 1975, 22, 401. (Review)
- ↑ Yamamura, S.; Nishiyama, S. Comp. Org. Syn. 1991, 8, 309-313.(Review)
- ↑ Boese R, Weiss HC, Blaser D (1999). "The melting point alternation in the short-chain n-alkanes: Single-crystal X-ray analyses of propane at 30 K and of n-butane to n-nonane at 90 K". Angew Chemie Int Ed. 38: 988–992. doi:10.1002/(SICI)1521-3773(19990401)38:7%3C988::AID-ANIE988%3E3.3.CO;2-S.
ar:ألكان bg:Алкани ca:Alcà cs:Alkany da:Alkan de:Alkane et:Alkaanid el:Αλκάνια eo:Alkano fa:آلکان fo:Alkan ko:알케인 hr:Alkani id:Alkana is:Alkanar it:Alcani he:אלקאן ku:Alkan la:Alcanum lv:Alkāni lt:Alkanas mk:Алкан ms:Alkana nl:Alkaan no:Alkaner nn:Alkan simple:Alkane sk:Alkán sl:Alkan sr:Алкан sh:Alkani su:Alkana fi:Alkaani sv:Alkan ta:ஆல்க்கேன் th:อัลเคน uk:Алкани