Acute myeloid leukemia natural history
|
Acute myeloid leukemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Acute myeloid leukemia natural history On the Web |
|
American Roentgen Ray Society Images of Acute myeloid leukemia natural history |
|
Risk calculators and risk factors for Acute myeloid leukemia natural history |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Raviteja Guddeti, M.B.B.S. [2], Carlos A Lopez, M.D. [3], Shyam Patel [4]; Grammar Reviewer: Natalie Harpenau, B.S.[5]
Overview
The natural history of acute myeloid leukemia involves the commencement of symptoms including fatigue, bleeding, and infection. Some patients will also present with disseminated intravascular coagulation in which bleeding and thrombosis occurs simultaneously. Complications of acute myeloid leukemia include infection, hemorrhage, venous thromboembolism, and therapy-related complications. The prognosis of acute myeloid leukemia is largely based upon on the European LeukemiaNet (ELN) classification system. The natural history of acute promyelocytic leukemia is also related to defective normal blood cell production, which include fatigue, bleeding, and infection. Complications include thrombosis and hemorrhage, which eventually occur in a significant proportion of patients. Early death is common and is related to bleeding complications. Therapy-related complications of acute promyelocytic leukemia include differentiation syndrome, QT interval prolongation, and cardiomyopathy. The prognosis of acute promyelocytic leukemia was previously poor, but the advent of arsenic trioxide and all-trans retinoic acid has rendered the prognosis to be far more favorable in the recent years.
Natural History
- Acute myeloid leukemia usually begins with a variety of symptoms including fatigue, bleeding, and infections (such as upper respiratory tract infection).
- complete blood count usually reveals a low white blood cell count, low hemoglobin, and/or low platelet count.
- Bone marrow biopsy is usually done to work up the abnormal laboratory values, and a diagnosis of acute myeloid leukemia is made.
- The median survival in the absence of treatment is typically 6-8 weeks.
- In patients with acute promyelocytic leukemia, in the first few days to weeks of the disease, there is a high risk of bleeding due to disseminated intravascular coagulation.[1]
- The median survival in the absence of treatment of acute promyelocytic leukemia is typically one week, due to bleeding complications contributing to mortality.[2]
- The high early mortality rate was previously a major part of the natural history of the disease, prior to the advent of rapid diagnostic and therapeutic interventions for this disease.[3]
- In areas of the United States with limited healthcare or highly specialized academic centers, bleeding diathesis continues to remain a major part of the natural history of the disease.
- Such bleeding complications include gingival bleeding (very common), bruising (very common), epistaxis, menorrhagia (less common).
- In areas of the United States with readily available healthcare and specialized academic medical centers, the natural history of the disease takes a favorable trajectory, as the cure rate is quite high if appropriate induction therapy is initiated.[3]
Complications
Common complications of acute myeloid leukemia and promyelocytic leukemia include:
Infection:
- Infection is the most common complication of acute myeloid leukemia. Infection arises due to impaired function of white blood cells. In patients with acute myeloid leukemia, most white blood cells are immature and cannot differentiate into mature neutrophils. The differentiation block prevents the body from producing infection-fighting cells. Patients are at increased risk for the following types of infections:
- Bacterial: Patients may require workup including imaging and blood cultures. Treatment includes intravenous antibiotics such as cefepime, piperacillin-tazobactam, or meropenem.
- Viral: Patients may require workup including imaging, serologies, and viral titers. Treatment includes anti-viral agents such as acyclovir.
- Fungal: Patients may require workup including imaging and fungal cultures. Treatment includes anti-fungal agents such as isovuconazole, posaconazole, or voriconazole.
Hemorrhage
- Acute myeloid leukemia, especially the promyelocytic sub-type, is frequently associated with bleeding caused by disseminated intravascular coagulation (DIC).
- Hemorrhagic and bleeding diathesis is the major cause of early complications that can lead to immediate death in patients with acute promyelocytic leukemia.
Venous thromboembolism
- Thrombus formation is a major cause of morbidity in acute promyelocytic leukemia.
- Thrombosis in the setting of acute promyelocytic leukemia is associated with a worse outcome compared to non-cancer-related thrombosis.[4]
- Studies have shown that nearly 80% of patients with venous thromboembolism and cancer die within 6 months of the diagnosis of venous thromboembolism.
- The reason for this correlation between thrombosis and death in acute promyelocytic leukemia is that thrombosis is a surrogate marker for disease progression.
- Procoagulants: There is increased production of procoagulant molecules such as thrombin from cancer cells. Furthermore, mucins and cytokines produced by malignant promyelocytes can induce endothelial cells to increase tissue factor production, and tissue factor functions in the extrinsic pathway to promote coagulation.
- Platelets: There is a increased platelet activation in acute promyelocytic leukemia.
- Fibrin: There is decreased fibrinolytic activity in acute promyelocytic leukemia, and this results in presence of excess fibrin. Fibrin is also known as factor I of the coagulation cascade and functions to binds platelets together via their GpIIb/IIIa receptors. This is one of the final steps in coagulation.
- Natural anticoagulants: There is decreased production of natural anticoagulants, and this results in increased propensity for thrombosis.
- Catheters: Central venous catheters can serve as a nidus for thrombosis since there is localized tissue and endothelial damage at the site of catheter insertion. and along the catheter within the body.[4] Patients with acute promyelocytic leukemia are more likely to have central venous catheters, compared to patients with other conditions, since chemotherapy usually requires the presence of a central catheter to be placed.
- Immobility: Patients with acute promyelocytic leukemia are frequently confined to a hospital bed during induction therapy, and venous stasis contributes to thrombosis. Obesity can also contribute to thrombosis.
- Erythropoiesis-stimulating agents: Patients with acute promyelocytic leukemia frequently have anemia. Some patients receive erythropoiesis-stimulating agents, such as erythropoietin, which can increase red blood cell production and exacerbate thrombotic complications.
- In a 2015 study from MD Anderson Cancer Center, it was shown that the annual incidence of venous thromboembolism, which includes deep vein thrombosis and pulmonary embolism, was 6.1-42%, which is the highest amongst all leukemia sub-types.[4]
- In contrast, the incidence of venous thromboembolism in chronic myeloid leukemia was only 1.5%.
| Disease | Thrombotic Incidence |
|---|---|
|
Acute myeloid leukemia |
3.7% |
|
Acute promyelocytic leukemia |
6.1-48% |
|
Chronic lymphocytic leukemia |
2.7% |
|
Acute lymphoblastic leukemia |
2.1-13% |
|
Chronic myeloid leukemia |
1.5% |
Therapy-related complications:
Treatment of acute myeloid leukemia can result in a variety of complications which are somewhat unique to the disease.
- Rash: Cytarabine-related rash is very common after induction chemotherapy. This can be treated with corticosteroids.
- Cerebellar toxicity: Patients treated with high-dose cytarabine (greater than 2-3g/m2) can develop cerebellar toxicity and thus require routine neurologic exams during consolidation chemotherapy.
- Conjunctival toxicity: Patients treated with high-dose cytarabine (greater than 2-3g/m2) can develop conjunctivitis and thus require routine ocular exams and prophylactic steroid eye drops during consolidation chemotherapy.
- Cardiomyopathy: Patients receiving chemotherapy with anthracyclines, such as idarubicin or daunorubicin, are at risk for short-term cardiac-related complications such as arrhythmias and long-term cardiac-related complications such as systolic dysfunction and heart failure. The highest risk of these complications occurs in patients with underlying cardiomyopathy such as congestive heart failure, atrial fibrillation, or other cardiac issues. The cardiotoxicity of anthracyclines is dose-dependent and generally irreversible.
- Differentiation syndrome: In patients with relapsed or refractory acute myeloid leukemia harboring the IDH2 mutation treated with enasidenib, differentiation syndrome can result. The incidence of differentiation syndrome overall after enasidenib treatment is about 10%, and the incidence of grade 3 or higher differentiation syndrome is 7%. It also occurs in patients with acute promyelocytic leukemia after treatment with all-trans retinoic acid.[5] This condition is characterized by weight gain, peripheral edema, hypoxia, dyspnea, renal failure, fever, and hypotension. The syndrome is thought to be due to systemic inflammation induced by the release of cytokines from malignant promyelocytes. This results in endothelial cell damage with resultant capillary leakage. Malignant promyelocytes are then able to adhere to tissue that is perfused by the microcirculation.[5] Patients with a high white blood cell count are at highest risk for differentiation syndrome, since all-trans retinoic acid will result in release of a large amount of cytokines if there is a high leukemia burden. Differentiation syndrome is a major complication that must be recognized early on, such that proper corrective measures can be taken. These include the use of dexamethasone 10mg PO twice daily, plus supportive treatment for any underlying respiratory distress. Diruesis may be needed to help eliminate excess fluid accumulation.
- QT interval prolongation: In patients with relapsed or refractory acute myeloid leukemia harboring the IDH1 mutation treated with ivosidenib, QT interval prolongation occurs in approximately 8% of patients. In patients with acute promyelocytic leukemia, arsenic trioxide can result in prolonged QT interval, which carries a risk for cardiac-related complications such as arrhythmias. Patients who are treated with arsenic trioxide must have routine electrocardiograms (EKGs) done to ensure that the corrected QT interval remains less than 500 milliseconds. In patients who are treated with concomitant chemotherapy and arsenic trioxide, such as patients with high-risk acute promyelocytic leukemia, there is a higher risk for cardiac-related complications. Chemotherapy and intravenous fluids can alter electrolyte such as potassium levels. Hypokalemia (low potassium) can exacerbate QT prolongation.
Prognosis
The European LeukemiaNet classification from 2017 represents the most recent prognostic scheme for acute myeloid leukemia.[6] This prognostic scheme is based on cytogenetic and molecular features of the disease. Prognostic categories as determined by European LeukemiaNet 2017 classification include favorable prognosis, intermediate prognosis, and poor prognosis.
| Name | Description |
|---|---|
| Favorable prognosis | Includes:
|
| Intermediate prognosis | Includes:
|
| Adverse prognosis | Includes:
|
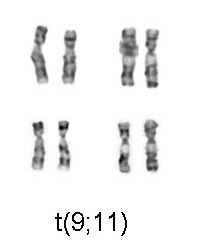
Acute myeloid leukemia is a curable disease; the chance of cure for a specific patient depends on a number of prognostic factors.[7] The first publication to address cytogenetics and prognosis was the MRC trial of 1998:[8][9][10][11]
Later, the Southwest Oncology Group and Eastern Cooperative Oncology Group,[12] and later still, Cancer and Leukemia Group B published other, mostly overlapping lists of cytogenetics prognostication in leukemia[13]
Prognosis of acute promyelocytic leukemia:
- Prior to the introduction of readily available diagnostics and targeted therapeutics, the prognosis of acute promyelocytic leukemia was previously very poor, especially in the early phase of the disease.
- The poor prognosis was due to high bleeding risk and death from hemorrhagic complications due to disseminated intravascular coagulation.
- Death typically occurs with a few days to weeks in the absence of treatment.
- The early death rate is estimated to be 17.3%, based on a large population-based analysis conducted in the United Stated between 1992-2007.[14] [2]
- The 5-year survival rate is only 30-40% after 5 years in younger patients.[5]
- In the current era of medicine (after the introduction of all-trans retinoic acid and arsenic trioxide), the prognosis of acute promyelocytic leukemia carries a much better prognosis.[3]
- Patients can achieve long-term, durable remission if treated appropriately in an expedited manner with medications such as all-''trans'' retinoic acid, arsenic trioxide, or cytotoxic chemotherapy.
- The current overall survival rate is 86-97%, and the complete remission rate is 90-100%.[5]
- In a multi-center study published in 2017 evaluating long-term outcomes of patients with acute promyelocytic leukemia, the complete remission rate was 96%.[15]
- Induction mortality is low at 4%.[15]
Other prognostic markers
- Antecedent myelodysplastic syndrome or myeloproliferative disorder: Acute myeloid leukemia which arises from a pre-existing myelodysplastic syndrome or myeloproliferative disease (so-called secondary AML) has a worse prognosis, as does treatment-related AML arising after chemotherapy for another previous malignancy. Both of these entities are associated with a high rate of unfavorable cytogenetic abnormalities.[16][17][18] In some studies, age > 60 years and elevated lactate dehydrogenase level were also associated with poorer outcomes.[19] As with most forms of cancer, performance status (i.e. the general physical condition and activity level of the patient) plays a major role in prognosis as well.
- Therapy-related leukemia: Acute myeloid leukemia which arises after receipt of chemotherapy for another type of cancer has a worse prognosis compared to de novo acute myeloid leukemia.
- Age: Older age portends a worse prognosis for patients with acute myeloid leukemia.
| Age group | 5-year overall survival[20] |
|---|---|
| 15-24 years old | 53% |
| 60-69 years old | 13% |
| 70-79 years old | 3% |
| 80 years and beyond | 0% |
Overall expectation of cure
- Cure rates in clinical trials have ranged from 20–45%;[21][22] however, it should be noted that clinical trials often include only younger patients and those able to tolerate aggressive therapies. The overall cure rate for all patients with acute myeloid leukemia (including the elderly and those unable to tolerate aggressive therapy) is likely lower. Cure rates for promyelocytic leukemia can be as high as 98%.[23]
References
- ↑ Franchini M, Lippi G, Manzato F (2006). "Recent acquisitions in the pathophysiology, diagnosis and treatment of disseminated intravascular coagulation". Thromb J. 4: 4. doi:10.1186/1477-9560-4-4. PMC 1402263. PMID 16504043.
- ↑ 2.0 2.1 Chen C, Huang X, Wang K, Chen K, Gao D, Qian S (2018). "Early mortality in acute promyelocytic leukemia: Potential predictors". Oncol Lett. 15 (4): 4061–4069. doi:10.3892/ol.2018.7854. PMC 5835847. PMID 29541170.
- ↑ 3.0 3.1 3.2 Coombs CC, Tavakkoli M, Tallman MS (2015). "Acute promyelocytic leukemia: where did we start, where are we now, and the future". Blood Cancer J. 5: e304. doi:10.1038/bcj.2015.25. PMC 4450325. PMID 25885425.
- ↑ 4.0 4.1 4.2 Vu K, Luong NV, Hubbard J, Zalpour A, Faderl S, Thomas DA; et al. (2015). "A retrospective study of venous thromboembolism in acute leukemia patients treated at the University of Texas MD Anderson Cancer Center". Cancer Med. 4 (1): 27–35. doi:10.1002/cam4.332. PMC 4312115. PMID 25487644.
- ↑ 5.0 5.1 5.2 5.3 McCulloch D, Brown C, Iland H (2017). "Retinoic acid and arsenic trioxide in the treatment of acute promyelocytic leukemia: current perspectives". Onco Targets Ther. 10: 1585–1601. doi:10.2147/OTT.S100513. PMC 5359123. PMID 28352191.
- ↑ Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T; et al. (2017). "Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel". Blood. 129 (4): 424–447. doi:10.1182/blood-2016-08-733196. PMC 5291965. PMID 27895058.
- ↑ Estey E (2001). "Prognostic factors in acute myelogenous leukemia". Leukemia. 15 (4): 670–2. PMID 11368376.
- ↑ Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. 1998 Oct 1;92(7):2322–33.
- ↑ Wheatley K, Burnett A, Goldstone A, Gray R, Hann I, Harrison C, Rees J, Stevens R, Walker H (1999). "A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties". Br J Haematol. 107 (1): 69–79. PMID 10520026.
- ↑ Slovak M, Kopecky K, Cassileth P, Harrington D, Theil K, Mohamed A, Paietta E, Willman C, Head D, Rowe J, Forman S, Appelbaum F (2000). "Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study". Blood. 96 (13): 4075–83. PMID 11110676.
- ↑ Byrd J, Mrózek K, Dodge R, Carroll A, Edwards C, Arthur D, Pettenati M, Patil S, Rao K, Watson M, Koduru P, Moore J, Stone R, Mayer R, Feldman E, Davey F, Schiffer C, Larson R, Bloomfield C (2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood. 100 (13): 4325–36. PMID 12393746.
- ↑ Slovak ML; Kopecky KJ; Cassileth PA; Harrington DH; Theil KS; Mohamed A; Paietta E; Willman CL; Head DR; Rowe JM; Forman SJ; Appelbaum FR Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000 Dec 15;96(13):4075–83.
- ↑ Byrd J, Mrózek K, Dodge R, Carroll A, Edwards C, Arthur D, Pettenati M, Patil S, Rao K, Watson M, Koduru P, Moore J, Stone R, Mayer R, Feldman E, Davey F, Schiffer C, Larson R, Bloomfield C (2002). "Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461)". Blood. 100 (13): 4325–36. PMID 12393746.
- ↑ Park J, Jurcic JG, Rosenblat T, Tallman MS (2011). "Emerging new approaches for the treatment of acute promyelocytic leukemia". Ther Adv Hematol. 2 (5): 335–52. doi:10.1177/2040620711410773. PMC 3573416. PMID 23556100.
- ↑ 15.0 15.1 Abaza Y, Kantarjian H, Garcia-Manero G, Estey E, Borthakur G, Jabbour E; et al. (2017). "Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab". Blood. 129 (10): 1275–1283. doi:10.1182/blood-2016-09-736686. PMC 5413297. PMID 28003274.
- ↑ Thirman M, Larson R (1996). "Therapy-related myeloid leukemia". Hematol Oncol Clin North Am. 10 (2): 293–320. PMID 8707757.
- ↑ Rowley J, Golomb H, Vardiman J (1981). "Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease". Blood. 58 (4): 759–67. PMID 7272506.
- ↑ Pedersen-Bjergaard J, Andersen M, Christiansen D, Nerlov C (2002). "Genetic pathways in therapy-related myelodysplasia and acute myeloid leukemia". Blood. 99 (6): 1909–12. PMID 11877259.
- ↑ Haferlach T, Schoch C, Löffler H, Gassmann W, Kern W, Schnittger S, Fonatsch C, Ludwig W, Wuchter C, Schlegelberger B, Staib P, Reichle A, Kubica U, Eimermacher H, Balleisen L, Grüneisen A, Haase D, Aul C, Karow J, Lengfelder E, Wörmann B, Heinecke A, Sauerland M, Büchner T, Hiddemann W (2003). "Morphologic dysplasia in de novo acute myeloid leukemia (AML) is related to unfavorable cytogenetics but has no independent prognostic relevance under the conditions of intensive induction therapy: results of a multiparameter analysis from the German AML Cooperative Group studies". J Clin Oncol. 21 (2): 256–65. PMID 12525517.
- ↑ Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z,Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2011, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014.
- ↑ Cassileth P, Harrington D, Appelbaum F, Lazarus H, Rowe J, Paietta E, Willman C, Hurd D, Bennett J, Blume K, Head D, Wiernik P (1998). "Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission". N Engl J Med. 339 (23): 1649–56. PMID 9834301.
- ↑ Matthews J, Bishop J, Young G, Juneja S, Lowenthal R, Garson O, Cobcroft R, Dodds A, Enno A, Gillett E, Hermann R, Joshua D, Ma D, Szer J, Taylor K, Wolf M, Bradstock K (2001). "Patterns of failure with increasing intensification of induction chemotherapy for acute myeloid leukaemia". Br J Haematol. 113 (3): 727–36. PMID 11380464.
- ↑ Sanz M, Lo Coco F, Martín G, Avvisati G, Rayón C, Barbui T, Díaz-Mediavilla J, Fioritoni G, González J, Liso V, Esteve J, Ferrara F, Bolufer P, Bernasconi C, Gonzalez M, Rodeghiero F, Colomer D, Petti M, Ribera J, Mandelli F (2000). "Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups". Blood. 96 (4): 1247–53. PMID 10942364.